Vaccines
Latest News
Latest Videos

CME Content
More News

The federal agency said that due to administrative constraints, they will not complete the review of mRNA-1345 by the PDUFA date of May 12.

Stating low demand, the company is removing their ChAdOx1-S [recombinant] (Vaxzevria) vaccine from the market.

Insights from the Annual Conference on Vaccinology Research (ACVR) on vaccine registries and assessing the safety of maternal and fetal health.

The investigational vaccine, mRNA-1345, developed for seniors has its FDA PDUFA date in a few days.
A study presented at ESCMID examines the efficacy and safety of the vaccine in older populations.

FDA-approves mpox vaccine, FDA grants EUA for at-home multiplex papid test, FDA grants PDUFA for GSK 5-in-1 meningococcal vaccine, and more this month from the FDA.

Despite the disproportionate effects of SARS-CoV-2 infection and severe outcomes on nursing home residents, less than 50% have received the updated 2023/2024 vaccine.
The study findings imply newly available prevention strategies for which older children—ages 2 to 5 years—are not currently eligible should be prioritized.
The vaccine’s developer, YS Biopharma, will begin its phase 1 clinical trial in the Philippines this summer.

The company’s investigational ABCWY vaccine candidate will be reviewed by the FDA by February 14, 2025.

The Centers for Disease Control and Prevention (CDC) and Detroit Health Department stress the importance of vaccination and surveillance.
New data demonstrates protection against new variants.
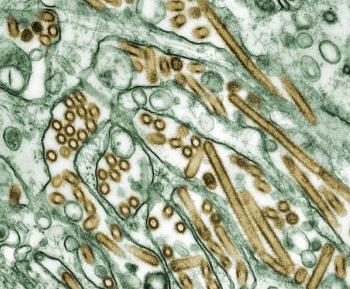
The origins of the recent H5N1 (avian flu) case in Texas remain unknown, but a researcher offers insights into transmission between animals and humans, the likelihood of more cases, vaccine availability, and treatment.

The company is going to submit their data to the FDA to seek approval for people within this age group.
A study on Italian vaccination rates and factors influencing Rotavirus vaccine acceptance.
Trial examines high-dose vs. standard-dose influenza vaccines.

As a potential alternative to antibiotic treatments, this investigational immunization offered protection for several years.

During the 2014-2016 African epidemic, investigational Ebola vaccines demonstrated safety and immunogenicity.

An FDA decision on the company’s mRNA-1345 is slated for May 12.

The RSV prefusion F protein–based maternal vaccine phase 3 trial was halted due to the risk of this adverse event.
Bavarian Nordic's JYNNEOS available to healthcare providers nationwide.

This very large study reviewed data, which showed people vaccinated for the virus had a substantial reduction of risk for these types of events.

The company's latest investigational vaccine induced a greater immune response compared to its FDA approved Spikevax product.

A large European study looked at a modern-day communication method and its influence on a public health initiative to increase immunizations.
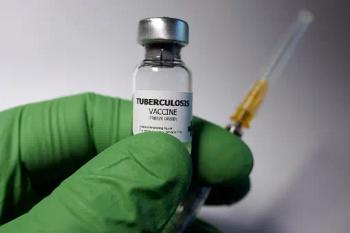
The Bill & Melinda Gates Medical Research Institute launches the M72/AS01E vaccine in South Africa to combat TB.