A Rare Pediatric Case: Recurrent Corynebacterium pseudotuberculosis Central Line–Associated Bloodstream Infection
To the authors' knowledge, this is the first reported case of C pseudotuberculosis bloodstream infection in an infant.
Final Diagnosis
Recurrent Corynebacterium pseudotuberculosis central line–associated bloodstream infection in an infant receiving parenteral nutrition
Abstract
Corynebacterium pseudotuberculosis is a gram-positive, facultative anaerobic pathogen commonly associated with disease in ruminant animals but is rarely reported in humans. We present what we believe to be the first documented case of C pseudotuberculosis central line–associated bloodstream infection in an infant.
The patient, a 7-month-old with short-gut syndrome requiring total parenteral nutrition via a central venous catheter, developed recurrent bacteremia with this rare pathogen. This case highlights diagnostic challenges, management considerations in immunocompromised pediatric patients, and the need for awareness of zoonotic and environmental organisms in nosocomial infections.
Introduction
Corynebacterium pseudotuberculosis is a zoonotic pathogen primarily known for causing caseous lymphadenitis in sheep and goats. Human infection is rare and typically linked to direct contact with infected animals or contaminated soil.1 Clinical manifestations in humans, when reported, range from lymphadenitis to ulcerative skin infections and, rarely, systemic disease. To our knowledge, this is the first reported case of C pseudotuberculosis bloodstream infection in an infant, particularly in the context of a central venous catheter and parenteral nutrition. The increasing use of long-term central venous access devices in pediatric patients with chronic gastrointestinal conditions places them at elevated risk for central line–associated bloodstream infections (CLABSIs). Although the usual suspects in CLABSIs are coagulase-negative staphylococci, Staphylococcus aureus, and Candida species, the presence of unusual organisms such as C pseudotuberculosis warrants careful microbiologic identification and thoughtful antimicrobial management.
Case Presentation
A 7-month-old male infant with a history of short-gut syndrome secondary to intestinal resection and long-term dependence on total parenteral nutrition (TPN) via a tunneled central venous catheter presented to an outside institution with a 2-day history of fever, watery diarrhea with streaks of blood, and increased fussiness. He had recently begun tasting pureed fruits and vegetables but remained reliant on TPN for the majority of his nutritional needs.
His past medical history was notable for multiple central line complications and gastrointestinal procedures. There were no recent sick contacts, travel, animal or farm exposures, or outdoor activities. His mother specifically denied that he had been exposed to soil, swimming pools, or grass. Initial laboratory evaluation demonstrated leukocytosis (23.8 x 10³/uL [reference range, 6.0-17.5]), neutrophilia (12.12 x 10³/uL [1.10-5.20]), eosinophilia (2.61 x 10³/uL [0.04-0.54]), anemia (9.1 g/dL [10.0- 15.0]), and elevated procalcitonin (1.51 ng/mL [< 0.50]).
Empiric antibiotic therapy with vancomycin and cefepime was initiated. Blood cultures drawn from both central and peripheral lines at the outside institution became positive for gram-positive bacilli within 24 hours. The pathogen could not be identified and was sent to a reference laboratory (Quest Diagnostics) for further analysis via DNA sequencing. It was later revealed to be C pseudotuberculosis. Upon transfer to our institution, the patient’s fever resolved within 24 hours, but blood cultures continued to yield growth.
Initial identification efforts, including Gram stain (gram-positive bacilli), catalase positivity, and negative motility, pointed to a Corynebacterium species. Two biochemical testing systems were used to identify the isolate: the Vitek 2 ANC card, an automated system that uses fluorometric technology to analyze metabolic activity, and the Remel RapID CB Plus system, a manual panel that relies on colorimetric biochemical reactions specific to Corynebacterium species. The Vitek 2 failed to produce a conclusive identification; whereas, the Remel system repeatedly identified the organism as C pseudotuberculosis. Due to the unusual result and discrepancy between the systems, the tests were repeated and confirmed the same identification. The isolate was found to be susceptible to vancomycin.
Treatment
Despite appropriate vancomycin therapy with pharmacokinetic monitoring with area under the curve (AUC)–guided dosing, blood cultures drawn from the central line remained positive for 4 days. On day 4, the central line was removed. A subsequent peripheral blood culture also grew C pseudotuberculosis, indicating possible hematogenous spread or residual bacteremia. The patient ultimately completed a 7-day course of intravenous vancomycin from the first negative culture. A new central line was placed after completion of therapy.
Thirty-eight days after finishing the antibiotic course, the patient was readmitted with fever, rhinorrhea, and fussiness. During the interim, he had undergone 2 central line repairs, an upper and lower endoscopy, and a gastrostomy tube exchange without febrile episodes or signs of infection. Repeat blood cultures from the central line again grew C pseudotuberculosis. This time, vancomycin was continued with the line left in situ. The patient was treated for 14 days from the first negative culture and showed no further evidence of relapse during that hospitalization. However, he continued to be hospitalized multiple times subsequently for line-related infections involving different pathogens.
Discussion
To our knowledge, this case represents the first reported episode of C pseudotuberculosis CLABSI in an infant. C pseudotuberculosis is typically associated with caseous lymphadenitis in ruminants and is an uncommon pathogen in humans. Reported human cases are typically associated with zoonotic or occupational exposures (ie, laboratory work) and most involve cutaneous or lymphatic infection. Its identification in a pediatric patient without known contact with animals or soil raises several important considerations in diagnosis, management, and prevention.
Pathogen Rarity and Environmental Reservoirs
C pseudotuberculosis is a facultative, intracellular, gram-positive bacillus, primarily known as a veterinary pathogen affecting sheep, goats, and horses.1 Human cases are rare, typically associated with direct animal contact or traumatic inoculation via soil-contaminated material. The absence of any such exposure in this infant, combined with strict home hygiene practices and no history of outdoor play, highlights a significant gap in understanding potential reservoirs or vectors for transmission. It is possible that environmental contamination of TPN materials or lapses in line care techniques played a role.
Microbiologic Challenges
The accurate and timely identification of C pseudotuberculosis is critical but difficult. In this case, automated platforms such as Vitek 2 failed to identify the organism; whereas the Remel RapID CB Plus system succeeded. The organism’s rare clinical appearance likely limits the sensitivity of many commercial identification systems. The case underscores the value of confirmatory biochemical and molecular testing (eg, sequencing), especially when standard diagnostics fail and clinical suspicion remains high.1 Routine clinical laboratory systems often have difficulty identifying rare species such as C pseudotuberculosis. In such scenarios, the use of reference laboratories, matrix-assisted laser desorption/ionization time of- flight mass spectrometry, or DNA sequencing is critical for accurate identification and targeted therapy.
Management Complexity
Persistent bacteremia despite appropriate vancomycin therapy warranted line removal, a cornerstone principle in managing CLABSI. Interestingly, even after line removal, the patient had continued positive blood cultures, suggesting a deepseated or biofilm-associated infection.2 Recurrence over a month later further supports the organism’s persistence and raises concerns about latent colonization or reintroduction, particularly given multiple line manipulations and procedures between episodes.
A prolonged vancomycin course with AUC-guided dosing was employed successfully both times. While no formal guidelines exist for treating C pseudotuberculosis in pediatric patients, susceptibility data supported continued vancomycin use. The decision to retain the line during the second episode was pragmatic, balancing the risk of procedural complications with infection control. Fortunately, this strategy was successful.
Literature Context and Implications
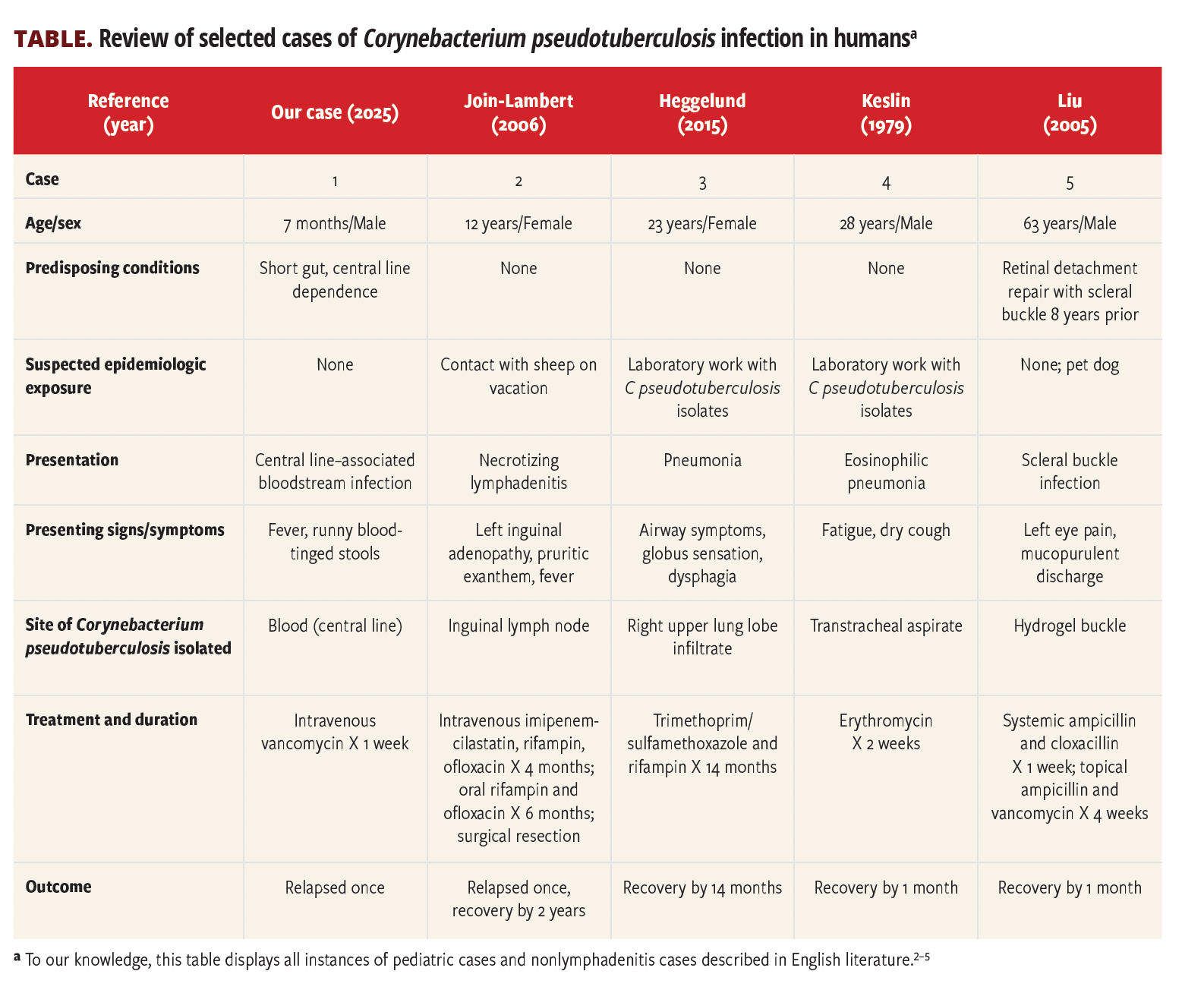
In published literature, human infections with C pseudotuberculosis are almost exclusively cutaneous or lymphatic in nature, with very few bacteremic cases—and none in infants. Most reports noted are nearly all systemic infections that involved immunocompromised adults, often with significant animal exposure.2-5 This case therefore broadens the known epidemiology and clinical spectrum of C pseudotuberculosis infections, especially in vulnerable hosts such as infants with intestinal failure.3,4
Further, this case supports a multimodal diagnostic approach, careful infection control practices for central lines, and a high index of suspicion for atypical organisms in patients with repeated line manipulations and broad-spectrum antibiotic exposure.
Conclusion
This case represents the first reported instance of C pseudotuberculosis bloodstream infection in an infant and highlights the complexity of diagnosing and managing rare pathogens in medically fragile pediatric patients. Clinicians should maintain a broad differential diagnosis in cases of persistent or unusual CLABSIs and be prepared to remove central lines when eradication is not achieved with appropriate antibiotic therapy. Awareness of environmental and zoonotic organisms, particularly in patients with long-term devices, is essential for prompt diagnosis and effective treatment.
References
1.Hiller E, Hörz V, Sting R. Corynebacterium pseudotuberculosis: Whole genome sequencing reveals unforeseen and relevant genetic diversity in this pathogen. PLoS One. 2024;19(8):e0309282. Published 2024 Aug 26. doi:10.1371/journal.pone.0309282
2.Join-Lambert OF, Ouache M, Canioni D, et al. Corynebacterium pseudotuberculosis necrotizing lymphadenitis in a twelve-year-old patient. Pediatr Infect Dis J. 2006;25(9):848-851. doi:10.1097/01.inf.0000234071.93044.77
3.Heggelund L, Gaustad P, Håvelsrud OE, et al. Corynebacterium pseudotuberculosis pneumonia in a veterinary student infected during laboratory work. Open Forum Infect Dis. 2015;2(2):ofv053. doi:10.1093/ofid/ofv053
4.Keslin MH, McCoy EL, McCusker JJ, Lutch JS. Corynebacterium pseudotuberculosis: a new cause of infectious and eosinophilic pneumonia. Am J Med. 1979;67(2):228-231. doi:10.1016/0002-9343(79)90395-4
5.Liu DT, Chan WM, Fan DS, Lam DS. An infected hydrogel buckle with Corynebacterium pseudotuberculosis. Br J Ophthalmol. 2005;89(2):245-246. doi:10.1136/bjo.2004.051698

Newsletter
Stay ahead of emerging infectious disease threats with expert insights and breaking research. Subscribe now to get updates delivered straight to your inbox.