Skin & Soft Tissue Diseases
Latest News
Video Series

Latest Videos
Podcasts
CME Content
More News

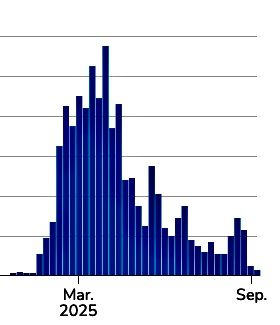
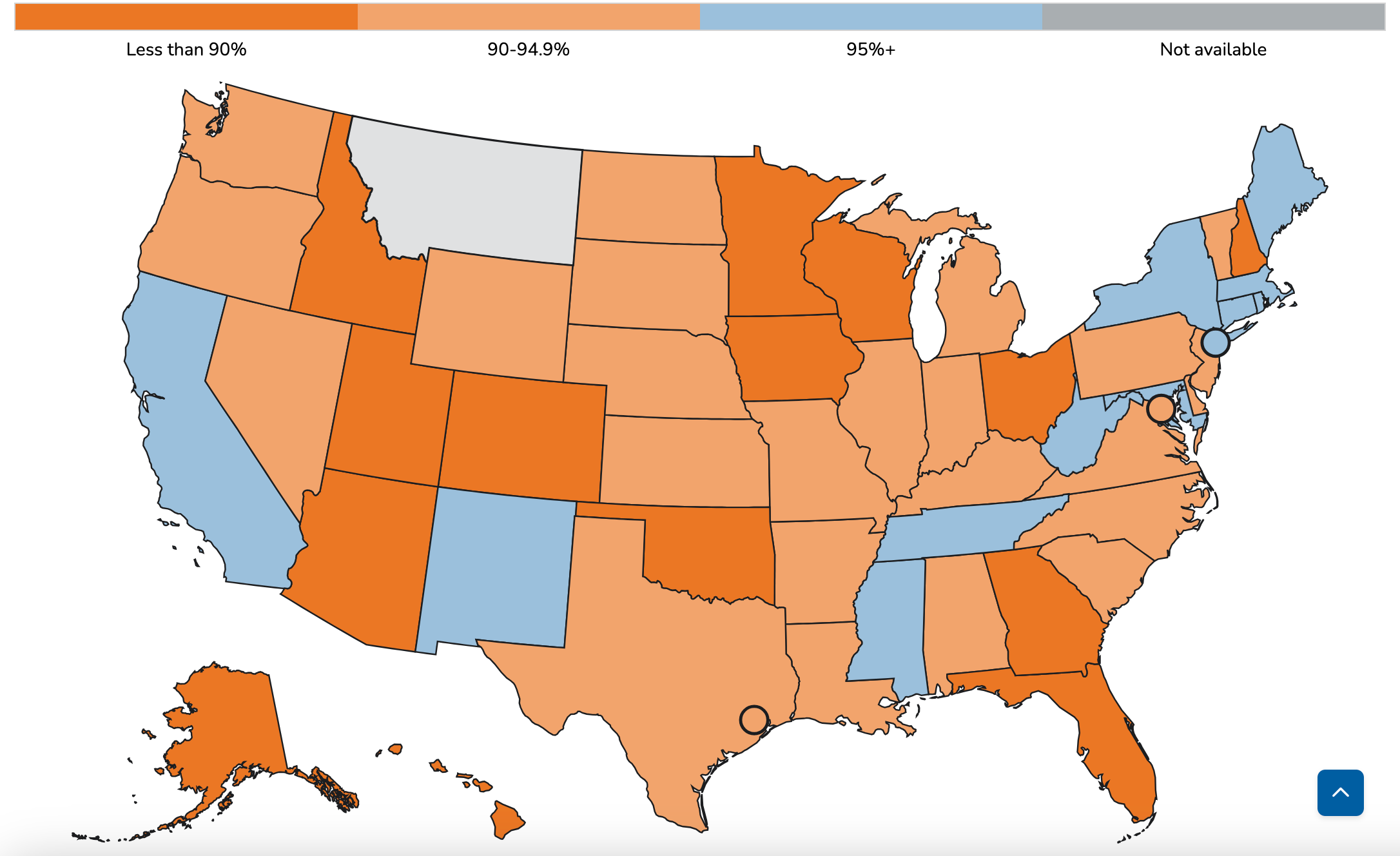
Ninety-four percent of cases were tied to 10 outbreaks, Texas leading with 624 cases, while Michigan and Montana reported the first outbreaks since 2019 and 1990, respectively.
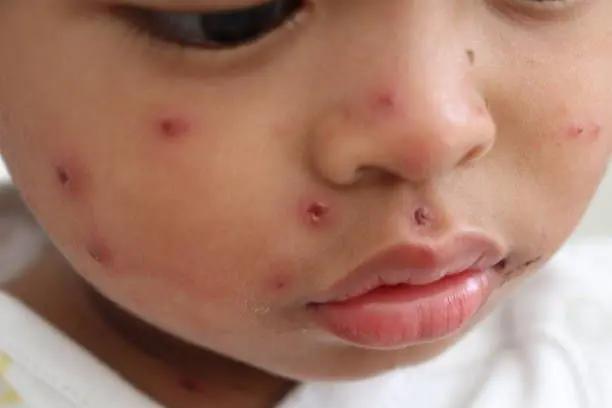
Amanda Truong, MD, PhD, provides her perspective on diagnosis, treatment, and environmental factors, considering her involvement in a recent human case.

Elke Wollants explains how wastewater surveillance in Belgium provided an early warning for measles outbreaks, offering insights into broader virus circulation.
The trial is being conducted by LimmaTech Biologics, and the first participants have been vaccinated.
Confirmed measles cases in Texas have risen from 24 to 58 in just six days, with 13 hospitalizations reported.

Oral omadacyline or linezolid was equally efficacious to intravenous dosing for acute bacterial skin infections, and associated with less cost and risks.
Sexual transmission was confirmed for nine of the 22 viruses studied, including Ebola, Zika, and mpox, making it a key factor in ongoing disease spread.
Interim findings from the STOMP trial reveal that tecovirimat does not significantly accelerate lesion resolution or reduce pain in mild to moderate mpox cases.

The investigational vaccine amezosvatein demonstrated low reactogenicity, supporting its potential as an alternative for shingles prevention in adults 50 and older.

This study revealed a significant decline in MSSA and a corresponding rise MRSA, particularly linked to COVID-19 social isolation measures.

Jeanine Thomas, president and founder of MRSA Survivors Network, talks about her personal battle with osteomyelitis, her work as an advocate, and the need for data and awareness to bring it to the forefront again.
As the WHO seeks Emergency Use Listing for new diagnostics due to rising cases in the Democratic Republic of the Congo, 50,000 doses of this vaccine will be donated to Central Africa.
Largest randomized trial of povidone iodine vs chlorhexidine gluconate could prompt WHO to recommend either over latter.

Study ranks antibiotic classes by risk for rare, life-threatening cutaneous reactions of Stevens-Johnson syndrome and toxic epidermal necrolysis.

In the current state of the mpox outbreak, individuals with advanced HIV face a higher risk of severe illness, making vaccination and targeted public health measures essential.

Bavarian Nordic is working to get their MVA-BN (Jynneos) mpox vaccine to those affected in African countries, as well as working towards a clinical trial for the younger pediatric population, and potential regulatory approval in Europe for adolescents.

Mpox virus evades the innate immune system by interfering with antiviral pathways signaling and interferon responses, increasing replication, and complicating outbreak management, especially in non-endemic regions.
Despite the differences in clinical presentation, MRSA strains remain susceptible to several key antibiotics, emphasizing the importance of targeted treatment and postoperative education in preventing MRSA infections.
We have complied some of our news stories and interviews from the last week to offer information on the differences between clade I and clade 2 mpox, why WHO declaring the global emergency is significant, looking at mpox in the United States, and more.

The vaccine, Jynneos, manufactured by Bavarian Nordic, is being shipped to Africa to address the ongoing mpox outbreak.
This is the very first such declaration by the Africa CDC since its inception in 2017.

In part 2 of this interview with Deborah Birx, MD, she highlights that ActivePure Technology provides a continuous decontamination solution that operates independently of manual intervention to reduce HAIs.

Part 1, Deborah Birx, MD explains photohydrolysis technology provides continuous, automated disinfection of air and surfaces effective in controlling MRSA and improving infection control practices in healthcare settings.

A large Veterans Health Administration (VA) outpatient study showed a large increase in MRSA resistance in one particular area of the United States and varying antimicrobial resistance patterns overall.

There is a high potential for ActivePure Medical’s technology to enhance patient safety and reduce healthcare costs by minimizing harmful pathogens in complex healthcare environments.