Virus Spillover and Emerging Pathogens Pick Up Speed
Infectious diseases are emerging at an alarmingly rapid pace, faster than any other time in human history.

Zoonotic diseases such as Ebola virus, rabies, Zika virus, African swine fever (ASF), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged into the human experience through an intricate and complex biological process known as spillover. Spillover of novel pathogens typically occurs through the intersection of the agent (eg, viruses, bacteria, parasites) with livestock, vectors, wildlife, or even the natural environment.
To make matters more complicated and concerning for public health and health care professionals, the spillover event is almost always invisible. Detecting emerging pathogens relies on strong public health surveillance systems and astute clinicians. Without well-resourced public health and health care infrastructure, such as advanced molecular epidemiology techniques, emerging pathogens and strains or variants of known pathogens can go unnoticed for long periods, resulting in delays in treatment and prevention measures.
The characterization and prediction of spillover intersections are of global importance, and it is critical to develop understanding of how, when, and where spillover events occur. The coronavirus disease 2019 (COVID-19) pandemic has spotlighted this issue; it is imperative to be better prepared for future pandemics.
Can we predict the next pandemic spillover event?
The jump of animal and environmental pathogens into human populations or other hosts is an ongoing biological “long game” that dates back thousands of years. From the current version of SARS-CoV-2, Ebola virus, measles virus, and HIV to historical epidemics such as plagues (eg, smallpox, cholera, and bubonic plague, also called the Black Death), pathogens have consistently found a way to move into the human population.1 In contrast to newer threats, rabies is one of the oldest described infectious diseases, likely with an ancient pedigree that predates most historical accounts.2 Regardless of the pathogen, this ongoing and longstanding “dance” between pathogens and susceptible hosts is a natural process, with the outcome of pathogen emergence in human populations likely comprising only a small percentage of spillover events between all animals.
With so many variables, such as complex pathogen life cycles, modes of transmission, and random susceptible hosts’ interaction, an initial spillover event resulting in ongoing transmission into a new host is relatively rare and usually an imperfect process. Most mammalian viruses lack the ability to infect humans. Moreover, should a natural zoonotic virus spillover into a human host, subsequent human-to-human transmission is often not possible or unsustainable. However, with enough opportunities, pathogens can adapt themselves to broaden their host range or evolve to sustain transmission in a more suitable host. Experts in pathogen spillovers believe that the rate of novel infectious disease emergence has increased in recent history.3-4
The dichotomy between host-range transmissibility bottlenecks and the increased rate of naturally occurring spillovers makes predicting what pathogen could trigger the next pandemic extremely difficult. Discovering an exact emerging pathogen within the global ocean of emerging and reemerging agents is a tall task, even for those equipped with advanced molecular diagnostics and epidemiologic technologies. That being said, there is a working knowledge of spillover events and how zoonotic and human intersections throughout the world can increase risk.1
The basics of the virus spillover event
Most spillover events are dead ends, meaning that the infectious agent could infect a novel host but is not capable of sustaining a transmission cycle within that new host. This happens frequently, particularly in areas where human and zoonotic populations overlap. Avian influenza is an excellent example of this. High pathogenicity avian influenza (HPAI) strains such as H5N1 and H7N9 have repeatedly spilled over into human hosts. Luckily, to date, there has not been a subsequent sustained human-to-human transmission in the spillover events from the current strains of HPAI; when human-to-human transmission has occurred, it has been limited.5
Unfortunately, viruses have an extensive history of learning to adapt to transmission within a new host. Occasionally a virus will acquire enough of the right mutations to infect a novel host and allow for subsequent and sustainable transmission in the host. HIV is an excellent example of this. The evolutionary ancestor of HIV is simian immunodeficiency virus (SIV), which has circulated in nonhuman primates for tens of thousands of years. Multiple independent spillover events of SIV occurred, but the virus was not fit to maintain transmission in a human host. That changed in the early 20th century when handling of bushmeat from an infected primate and blood-to-blood contact with the handler was believed to have occurred. This resulted in the initial spillover of a variant of SIV that could sustain itself in a novel host, humans, which led to the HIV pandemic.6
What about emerging and reemerging pathogens?
Emerging and reemerging pathogens occur and sustain themselves through a zoonotic vehicle. Zoonotic disease emergence arises as a consequence of transmission of a frequently multihost pathogen from an animal host (direct or indirect transmission) to a human, with or without onward human-to-human transmission and establishment. In most instances, a zoonotic reservoir is defined as 1 or more epidemiologically linked and intersecting animal populations or environments in which a pathogen can be maintained and transmitted to humans and, more broadly, domestic animals.1
Some of the most-studied viruses are those that intersect with zoonotic and human populations. Studies of RNA viruses (eg, Ebola virus, influenza, Lassa fever, rabies, coronaviruses, HIV) have provided insight into how they might be evolutionarily predisposed to zoonoses due to a predisposition toward evolvability and host switching.1 Results of a study by Johnson et al demonstrated a number of key rules, perhaps most importantly that viruses with greater host plasticity in animal reservoirs are more likely to demonstrate human-to-human transmissibility.7
Some virus families, such as arenaviruses (eg, Lassa fever) and filoviruses (eg, Ebola and Marburg viruses), are predisposed to human-to-human transmission. Correspondingly, some groups of mammals are common reservoirs of zoonotic viruses, including rodents (especially for arena- and bunyaviruses), primates (retroviruses), and bats (coronaviruses, paramyxoviruses, and rhabdoviruses).1 Spillover pathways also can vary. Hunting is a notable source of exposure to nonhuman primate viruses, and the proximity of homes to fields is a significant driver for exposure to rodent-borne viruses.8
It is almost impossible to predict the next big emerging or reemerging pathogens. However, there are factors that can contribute to an infectious agent becoming problematic for humans. These drivers come from genetic influences, environmental influences, and sociological behaviors. Some examples of potential spillover drivers are:
- An agent expanding to new geographic locations or new populations (eg, Vibrio populations and West Nile virus)
- An infectious agent that exhibits broad host-ranges (eg, coronaviruses and influenza viruses)
- A sustained zoonotic transmission cycle with nearby human population centers (eg, retroviruses and rhabdoviruses)
- A previously known agent(s) whose role in specific diseases have previously gone unrecognized (eg, herpesviruses)
- A family, genus, or species with a documented history of previous spillover events (eg, HPAI and Yersinia)
- Re-emerging infectious diseases with an incidence of disease that significantly declined in the past but have since reappeared (eg, measles).9
As the recent COVID-19 pandemic and prior emergence of pathogens have shown, there will be a “next” pandemic (or at least epidemic) pathogen. Investigators feel strongly there will be a SARS-CoV-3 pathogen.1 Additionally, variants of SARS-CoV-2 have quickly spread, demonstrating that novel phenotypes from a singular virus can take hold when multicontinental transmission rates are high.10
There are clues in recent history to what some of the more likely emergent pathogens will be. For example, 1 instance of a tropical disease that has spread recently into new areas due, at least in part, to changing climate is the chikungunya virus. As with dengue virus, it is transmitted by the Aedes mosquito, including the Aedes albopictus “tiger mosquito.” Chikungunya virus was previously confined to tropical regions around the Indian Ocean. However, in the past decade, the virus has been reported in countries in Europe, Asia, Africa, and the Americas, achieving sustained transmission.
Another example is the Zika virus, which burst onto the international scene through its association with a birth defect known as microcephaly. The Ebola virus epidemic that emerged in 2014 in West Africa illustrates how a virus that previously affected only small groups of individuals, perhaps a few hundred, can sweep rapidly through an area to affect tens of thousands and become extremely difficult to contain. In February, health authorities in Guinea declared an outbreak of Ebola in the rural community of Gouéké in Nzérékoré prefecture after 3 cases of Ebola were confirmed by the national laboratory, marking the first time the disease has been reported in the country since an outbreak ended in 2016.11
One of the more current worrisome emerging pathogens is the ASF virus. ASF is a highly contagious and deadly viral disease that affects domestic and wild pigs. Currently, ASF is not a threat to human health and cannot be transmitted from pigs to humans.12
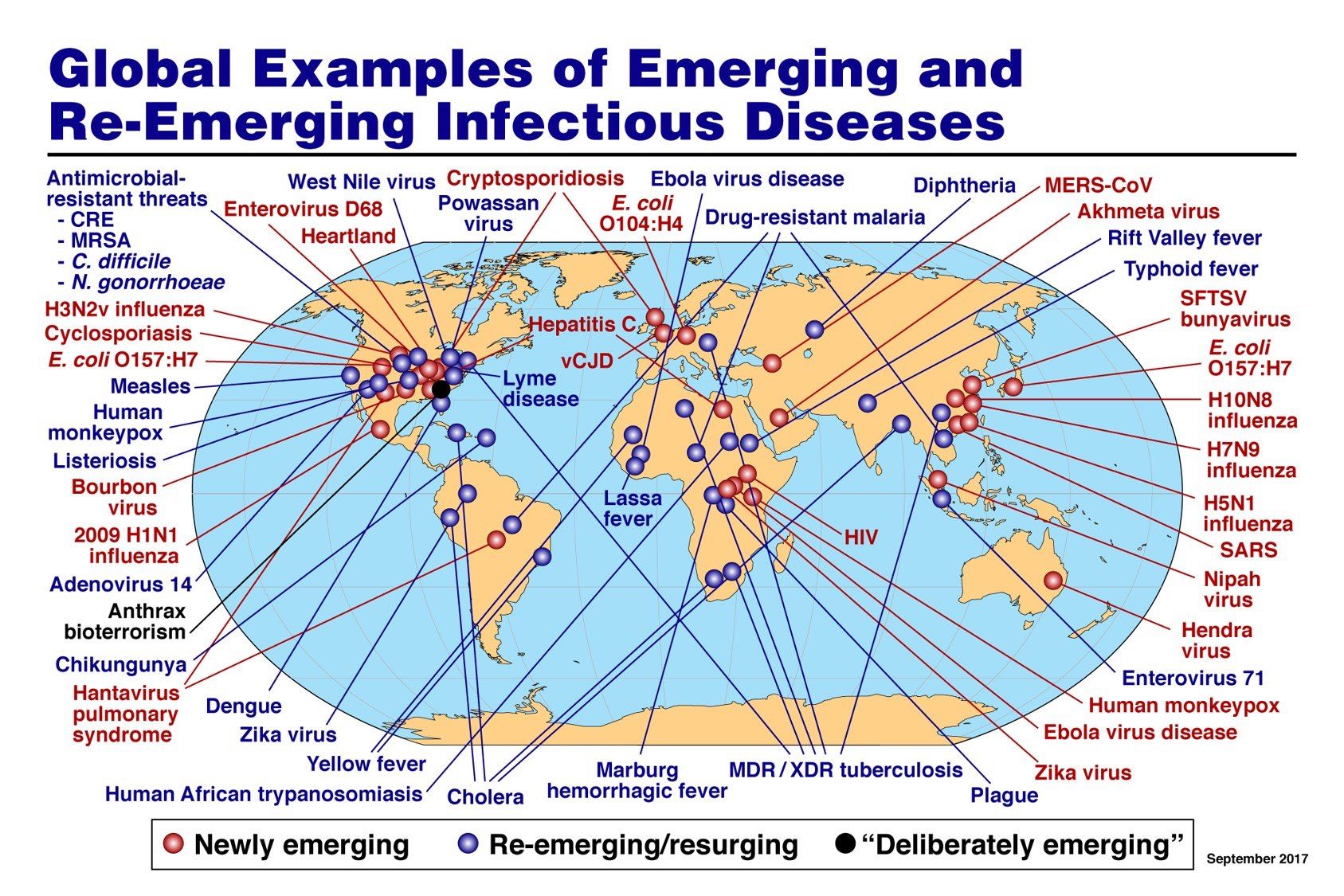
But we are always watching for the next emerging human pathogen (Figure).
Rodney E. Rohde, PhD, SM(ASCP), SVCM, MBCM, is professor and chair of the Clinical Laboratory Science Department, College of Health Professions, at Texas State University in San Marcos and an associate adjunct professor of biology at Austin Community College in Texas. He is a board certified specialist in virology, microbiology, and molecular biology with the American Society for Clinical Pathology.
Ryan P. McNamara, PhD, is a virologist and research associate at the University of North Carolina at Chapel Hill School of Medicine.
References
- Alexander KA, Carlson CJ, Lewis BL, et al. The ecology of pathogen spillover and disease emergence at the human-wildlife-environment interface. In: Hurst CJ, (eds). The Connections Between Ecology and Infectious Disease. Cham Springer; 2018:267-298. doi:10.1007/978-3-319-92373-4_8
- Rohde RE, Rupprecht C. Update on lyssaviruses and rabies: will past progress play as prologue in the near term towards future elimination? Fac Rev. 2020;9:9. doi:10.12703/b/9-9
- Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990-993. doi:10.1038/nature06536
- Johnson CK, Hitchens PL, Pandit PS, et al. Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc R Soc B. 2020;287(1924):28720192736. doi:10.1098/rspb.2019.2736
- McNamara R, Rohde RE. The imminence of the next SARS outbreak. Smerconish. January 14, 2021. Accessed February 17, 2021. https://www.smerconish.com/exclusive-content/the-imminence-of-the-next-sars-outbreak
- Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1(1):a006841. doi:10.1101/cshperspect.a006841
- Johnson CK, Hitchens PL, Evans TS, et al. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci Rep. 2015;5:14830. doi:10.1038/srep14830
- Dearing MD, Dizney L. Ecology of hantavirus in a changing world. Ann N Y Acad Sci. 2010;1195:99-112. doi:10.1111/j.1749-6632.2010.05452.x
- What are emerging infectious diseases? Baylor College of Medicine. Accessed February 17, 2021. https://www.bcm.edu/departments/molecular-virology-and-microbiology/emerging-infections-and-biodefense/emerging-infectious-diseases
- Emerging SARS-CoV-2 variants. Centers for Disease Control and Prevention. Updated January 28, 2021. Accessed February 17, 2021. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/scientific-brief-emerging-variants.html
- New Ebola outbreak declared in Guinea. World Health Organization. February 14, 2021. Accessed February 17, 2021. https://www.afro.who.int/news/new-ebola-outbreak-declared-guinea
- African Swine Fever (ASF). US Department of Agriculture. November 5, 2020. Accessed February 17, 2021. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/swine-disease-information/african-swine-fever/seminar

Newsletter
Stay ahead of emerging infectious disease threats with expert insights and breaking research. Subscribe now to get updates delivered straight to your inbox.
