
A small study found physical health problems lingered 1 year after their hospital stay.
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

A small study found physical health problems lingered 1 year after their hospital stay.

In a new federal survey, as many as 3 in 10 US adults have had the condition at some point.

This annual event hosted by The Peggy Lillis Foundation (PLF) released their full agenda for advocacy and education that will be happen next week in Washington DC.
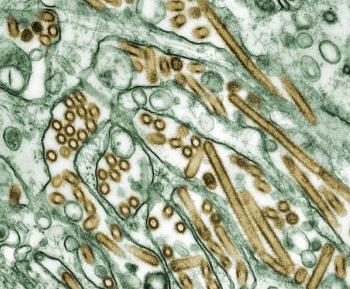
The origins of the recent H5N1 (avian flu) case in Texas remain unknown, but a researcher offers insights into transmission between animals and humans, the likelihood of more cases, vaccine availability, and treatment.

The company is going to submit their data to the FDA to seek approval for people within this age group.

The federal agency has given the nod for dolutegravir/lamivudine (Dovato) for teens living with the virus and offers a novel option for patients.

As a potential alternative to antibiotic treatments, this investigational immunization offered protection for several years.

The case involves a man who had contact with wild monkeys.

During the 2014-2016 African epidemic, investigational Ebola vaccines demonstrated safety and immunogenicity.

An FDA decision on the company’s mRNA-1345 is slated for May 12.

The RSV prefusion F protein–based maternal vaccine phase 3 trial was halted due to the risk of this adverse event.

The case was confirmed in Texas and the person was in contact with cows. CDC says the risk for infection remains low.

This very large study reviewed data, which showed people vaccinated for the virus had a substantial reduction of risk for these types of events.

The company's latest investigational vaccine induced a greater immune response compared to its FDA approved Spikevax product.
In today’s world of sophisticated, complex tests, an evolving relationship between laboratory professionals and clinicians can aid in providing a quicker diagnosis and help to achieve better patient outcomes.

The assay, the cobas Malaria test, is manufactured by Roche, and will be available in the United States at the end of Q2 2024.

The authorization was given to Invivyd’s pemivibart (Pemgarda), and it is indicated for adults and adolescents with moderate-to-severe immune compromised conditions.

This week learn more about CDC's strategies to reduce waterborne diseases; the US change to a trivalent influenza vaccine; new data for Merck's pneumococcal vaccine; and how PCR testing can influence a patient diagnosis and outcome.

A large European study looked at a modern-day communication method and its influence on a public health initiative to increase immunizations.
The company’s investigational vaccine, V116, is a conjugate vaccine and demonstrated immunogenicity for all 21 serotypes across the studied adult groups.

Today serves as a reminder to everyone that there are still no diagnostic tests or FDA-approved treatments for the post-viral condition.

The antifungal’s designation ensures its market viability and gives the youngest pediatric patients a therapy for health conditions that have been associated with high mortality rates.

A new study shows how correcting misinformation in a certain way can change patients’ attitudes towards vaccination.
An experienced measles clinician offers insights on the increase of incidence rates, preventative measures, and how vitamin A treatment can help those with the disease.
State health officials did not disclose the origin of transmission.
The first-in-class entry inhibitor delivered therapeutic benefits over a long study period for patients with chronic infection.

A team developed and validated a new analytic method to quantify omadacycline and its epimerization in stool to facilitate microbiome research.

The therapy, lenacapavir, showed sustained virological suppression and safety over a 2-year period in this treatment-challenged patient population.

Both the use of a vaginal ring or oral pre-exposure prophylaxis were found to be safe for HIV prevention throughout pregnancy.

Biomarkers associated with mortality differed by sex and there may be distinct pathophysiologic mechanisms that account for the increased risk seen in females, according to investigators.