
Ken Duncan, PhD, discussed the launch of a $50 million initiative focused on developing new drugs for critical pathogens contributing to antimicrobial resistance.

Ken Duncan, PhD, discussed the launch of a $50 million initiative focused on developing new drugs for critical pathogens contributing to antimicrobial resistance.

This region of Africa, which has been beset by mpox, malaria, Ebola, and Marburg disease is also seeing a mysterious illness with several hundred reported cases in 2 villages in Africa.

Todd Riccobene, PhD, senior scientific director, Anti-Infectives and Infectious Diseases, US Medical Affairs + Health Impact at AbbVie provides more information on the newly approved antibiotic combination for these infections.

James Ford, MD, MAS, discusses the evidence supporting SEP-1’s impact on sepsis mortality and suggests alternative approaches focused on outcome-based measures in sepsis management.

Tina Tan, MD, FIDSA, FPIDS, FAAP, Infectious Diseases Society of America (IDSA) president discusses the changes and offers a glimpse of what the US can expect in terms of limited access to new vaccines, increased incidence rates of disease, and new public health policy regarding immunizations.

Jeffrey Freiberg, MD, PhD review of the SABATO trial on oral antibiotics for S aureus bacteremia and a comparison of mupirocin and iodine treatments for MRSA decolonization.

The World Health Organization (WHO) reports on the vaccine uptake in that region, and the Global Polio Eradication Initiative reports that some countries recording new cases.

Jaime Garcia-Iglesias, PhD discusses community involvement, holistic benefits, and strategies for equitable rollout of doxycycline post-exposure prophylaxis.

The company announced its monoclonal antibody, pemivibart (Pemgarda), was denied the emergency use authorization (EUA) for treatment of mild-to-moderate COVID-19 for immunocompromised persons.

Jeffery Freiberg, MD, PhD review on key trials and challenges from 2024 in the fight against antibiotic resistance in complicated and uncomplictaed UTIs, including cefepime-taniborbactam and gepotidacin.

This week, the FDA set a target date for lenacapavir’s approval, as rising cases of dengue, measles, and other emerging threats continue to spread, alongside a dairy worker transmitting avian flu to his cats.

Chinese researchers say this newly discovered virus can be transmitted to humans much the way COVID-19 did.

The fixed-dose combination will be used outside the EU to treat soil-transmitted helminths and lymphatic filariasis.

Jeff Freiberg, MD, PhD, on the rising emerging infection concerns of dengue, mpox, measles, and H5N1 influenza in clinical practice.

Two cats became severely ill, and both owners declined to be tested.

A new study published in JAMA Network Open points out that immunization also occurred in individuals who did not receive routine prenatal or infant vaccines.

Andrew Engeli explains Ginkgo's role in the €24 million EU-Funded RANGER project for emerging infectious disease surveillance and response.

In a novel, natural experiment associated with age and the antiviral, investigators were able to see the effects of the therapy in a group of adults considered to be more at risk for hospitalization and death.

Sean Ong discusses how persistent fever beyond 72 hours emerges as a stronger predictor of mortality in MRSA bacteremia compared to CRP and WBC counts.

Oliver A Cornely shares guidance on tackling Candida infections in an evolving landscape, including rising resistance and taxonomic changes.

The company’s investigational gel, RECCE 327, met primary and secondary endpoints, and it plans to progress to a phase 3 trial.

Elke Wollants explains how wastewater surveillance in Belgium provided an early warning for measles outbreaks, offering insights into broader virus circulation.
The trial is being conducted by LimmaTech Biologics, and the first participants have been vaccinated.
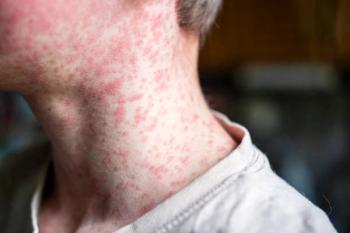
Confirmed measles cases in Texas have risen from 24 to 58 in just six days, with 13 hospitalizations reported.

The Prescription Drug User Fee Act (PDUFA) date is set for June 19. And if it is approved, it would be the first and only twice-yearly HIV pre-exposure prophylaxis (PrEP).

Emily Ricotta, PhD, MSc outlines the importance of involving affected populations and refining data collection methods to improve early decision-making during epidemics

A state official says the woman is hospitalized in another state, and had preexisting health conditions that can make people more vulnerable to illness.

Koos Korsten, MD, PhD, MSc discusses how clinical context, including prevalence and outbreak situations, influences the choice between PCR and culture-based diagnostics for Candidozyma auris.

The company's trivalent vaccine (mRNA-1403) was being studied in a phase 3 trial.