Vaccines
Latest News
Video Series

Latest Videos
Shorts





Podcasts
CME Content
More News

Although the declared COVID-19 pandemic public health emergency has passed, the CDC reports continued high numbers of illnesses, medical visits, hospitalizations and deaths.

Valneva has voluntarily withdrawn its US regulatory applications for its chikungunya vaccine, IXCHIQ, after the FDA suspended the license and placed the program on clinical hold pending investigation of a newly reported serious adverse event.
Evidence supporting the second of two licensed pentavalent meningococcal vaccines as an alternative to separate quadrivalent (ACWY) and serogroup B (MenB) vaccines is described and qualified in a recent MMWR.

The COVID-19 pandemic intensified long-standing problems in scientific production and communication—driven by perverse academic incentives, peer review strain, media hype, and growing misuse of AI—highlighting the urgent need for stronger critical appraisal skills across research and medicine.

This week, listen in on commentary around the changes to the childhood vaccine schedule, read SIDP's column on the next-generation antifungals as well as combination therapeutics for Candida auris, and the Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing breakpoint recommendations.

With rates of influenza vaccination in decline, two health systems evaluated interventions for prompting clinicians to order, and patients to obtain flu shots.

New federal guidance recommends childhood vaccines for 11 diseases, downsizing immunization protection from the previous list of 18 diseases.
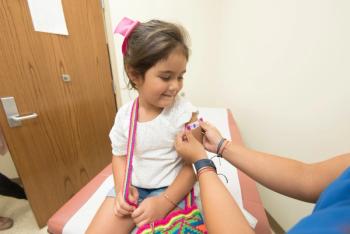
A delay in providing standard infant vaccinations is associated with children not receiving MMR (measles, mumps, rubella) vaccine by 2 years of age.

A series of politically driven actions in 2025—including defunding mRNA research, dismantling vaccine advisory bodies, and restricting CDC recommendations—has undermined US vaccine access and pandemic preparedness, but professional societies and some states are stepping in to preserve evidence-based public health.

The recent CDC ACIP meetings and subsequent actions show a path for which the current Health and Human Services department wants to roll back access to vaccines for this virus and other vaccines.

The single-source award will fund a large randomized trial evaluating mortality, morbidity, and developmental outcomes following the hepatitis B birth dose, amid ongoing debate over non-specific vaccine effects.
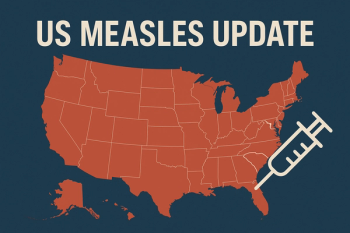
CDC reports over 1,900 confirmed cases and 49 outbreaks nationwide, with recent surges reported in South Carolina, Utah, Arizona, and Connecticut amid declining childhood vaccination coverage.

This new recommendation relates to infants born to women who test negative for hepatitis B. This is a departure from the federal agency’s previous recommendation and comes on the heels of the ACIP’s recent meeting.
An impending deadline is coming up in early 2026 that could cause the country to lose its status. However, this can be reversible and unnecessary infections, severe disease, and deaths can be avoided. Rodney Rohde, PhD, talks about incidence rates, how we got here, and strategies to increase immunizations.

Reports are surfacing the federal agency is going to review products already reviewed and approved.

House Lawmakers Probe FDA Over COVID-19 Vaccine Death Claims and Proposed Vaccine Regulation Changes
Questions raised about unverified FDA claims linking COVID-19 vaccines to pediatric deaths, and concerns that proposed vaccine testing requirements could delay access to key immunizations.

Lacking any safety data showing potential serious adverse effects, the committee decided to move forward with the recommendation “that the initial dose is administered no earlier than 2 months of age.”

Due to repetitive and confusing language, the committee decided to amend the votes for clarity and will vote on 3 new recommendations on Friday.

Anticipating possible constraints on the approval of COVID-19 vaccines and their removal from the CDC pediatric vaccine schedule, a medical ethicist and legal scholar consider off-label vaccination.
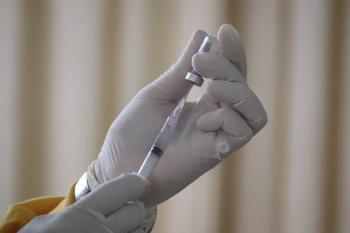
New Centers for Disease Control and Prevention (CDC) data show rising early-season influenza activity, moderate 2024–2025 vaccine effectiveness, and updated 2025–2026 recommendations that include trivalent, thimerosal-free single-dose formulations and expanded access to FluMist.

James Conway, MD, describes how full vs partial repeal of nonmedical school-entry exemptions is associated with kindergarten vaccination and exemption trends.

Large international study reports higher A-strain protection and acceptable safety profile for quadrivalent RNA influenza vaccine.

A review of vaccine safety and effectiveness conducted by the Vaccine Integrity Project, independent of the CDC's ACIP, reflects its stated purpose as "dedicated to safeguarding vaccine use in the US."

Infectious disease specialists conducted a review of vaccine safety and effectiveness independent of the ACIP to inform vaccinating against COVID-19, RSV, and influenza during this ongoing respiratory virus season.

Seven months of clinical data corroborated emergency authorization of 2024-2025 mRNA COVID-19 vaccines for circulating JN.1 subvariants.