Long-term clinical and economic benefits of an inclusive national HCV program targeting mothers and infants, two often overlooked populations.

Long-term clinical and economic benefits of an inclusive national HCV program targeting mothers and infants, two often overlooked populations.
Although the TB pipeline has several new agents in various phases, challenges to antimicrobial development remain. Therefore treatment adherence remains paramount.

Theratechnologies' new tesamorelin formulation provides a simplified, long-acting dosing regimen to effectively manage excess abdominal fat in adults with HIV-associated lipodystrophy.

A new analysis raises concerns over the stoppage of funding for this long-standing program, which could have major impacts on global public health for years to come.
Study uncovers the role of ploidy plasticity in fungal adaptation to fungicides, highlighting risks for human health and agricultural systems.

The 15th anniversary of the Affordable Care Act is overshadowed by threats to HIV prevention efforts, including a looming Supreme Court case that could eliminate no-cost access to preexposure prophylaxis and other preventive services. Simultaneously, deep federal cuts and restructuring have destabilized critical public health infrastructure, jeopardizing decades of progress in the fight against HIV.

Key updates from the Centers for Disease Control and Prevention on influenza activity, hospitalizations, pediatric deaths, and current trends across the United States.

A child who was not vaccinated succumbed to measles pulmonary failure. Additionally, increased cases and hospitalizations continue to grow greatly in the state.
One hospital related the evolution of resistant Acinetobacter baumannii and Pseudomonas aeruginosa in its ICU to the COVID-19 pandemic.

Development of a monoclonal antibody targeting mucormycosis, WHO’s call for improved fungal diagnostics and therapies, Bayesian dosing to optimize antimicrobial therapy, and more.

Environmental contamination plays a larger role than previously recognized, prompting calls for updated infection control strategies.

Patients with suspected sepsis in the emergency department are twice as likely to survive at 28 days when antibiotics are started within 1 hour.

Sharmeen Roy, PharmD, BCPS, on artificial intelligence (AI) in improving decision-making, reducing adverse drug events on National Adverse Drug Event Awareness Day, and upcoming research on pediatric dosing at the the Congress of the European Society of Clinical Microbiology and Infectious Diseases.

Andrew Aronsohn, MD, associate professor of Medicine, University of Chicago Medical Center, discusses the hepatitis C (HCV) screen and treat plan that looks to utilize a one visit approach to get people tested and on medication in the same patient encounter.

Sharmeen Roy, PharmD, BCPS, discusses the role of AI, real-world evidence, and clinician oversight in optimizing medication dosing for ICU patients.

Lumen Bioscience’s LMN-201 was considered safe and well tolerated, and there were no severe dose-related or serious adverse events reported.

Sharmeen Roy, PharmD, BCPS, discusses the role of Bayesian dosing, therapeutic drug monitoring, and hospital resource allocation.

Indwelling devices and previous antibiotic exposure increased the risk for these infections.

In a new study, Marc Ka-Chun Chong, PhD discusses the antiviral's impact on survival and hospitalization outcomes.
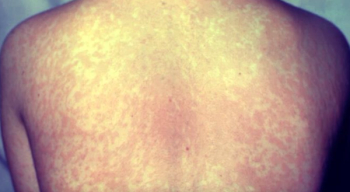
Ohio is now dealing with an outbreak, and the Centers for Disease Control and Prevention (CDC) reports nearly 500 total cases in the US.

Ashraf S. Ibrahim, PhD, outlines safety studies and the potential of this antibody therapy for treating this rare fungal infection, which is seemingly becoming less rare.

Clinical-stage company, BiomX, said its therapy, BX211, was found to be safe and efficacious for this infection that was associated with Staphylococcus aureus.

Novavax’s COVID-19 vaccine, Merck’s RSV antibody, and Innoviva’s gonorrhea antibiotic await regulatory decisions this quarter.

The organization's reports discuss the small number of newly approved treatments, the limited, late-stage antifungal pipeline, and challenges around diagnostics for this class of infections.

Ashraf S. Ibrahim, PhD, details over 20 years of research on a spore-coating protein and the development of VX-01 to block fungal tissue invasion.

Ashraf S. Ibrahim, PhD, discusses how VX-01 prevents fungal invasion of blood vessels and enhances immune response in immunocompromised patients.

New 21-valent conjugate vaccine authorized to combat invasive pneumococcal disease and pneumonia in individuals aged 18 and older.

Tina Tan, MD, FIDSA, FPIDS, FAAP, Infectious Diseases Society of America (IDSA) president, discusses the potential implications these layoffs will have on clinicians and overall public health.

The success of community-based point-of-diagnosis HCV treatment in the NOW trial was attributable to collaborative care with an integrated pharmacy team.

The Visby Medical Women’s Sexual Health Test can screen for chlamydia, gonorrhea, and trichomoniasis.