In patients with hepatitis D (HDV) and compensated cirrhosis, a newer antiviral therapy, bulevirtide (Hepcludex), proved to be efficacious and safe to patients.

In patients with hepatitis D (HDV) and compensated cirrhosis, a newer antiviral therapy, bulevirtide (Hepcludex), proved to be efficacious and safe to patients.
Investigators reviewed the current literature on the utility of oral vancomycin prophylaxis (OVP), and offered guidance for when this approach may be beneficial.

Ensitrelvir is an investigational 3CL protease inhibitor that reduced COVID-19 illness duration by 1 day.
Aaron Ferguson’s diagnosis completely changed his life’s path. Since then, he has helped others overcome their addictions and get hepatitis C (HCV) treatment. In his home state of Texas, a new health initiative is looking to get more people into the continuum of care for this curable virus.
The CDC’s recent warning of the rising incidence rates of the fungal infection is cause for concern, but understanding which patients and settings are at a higher risk is equally as important. The lead author in the CDC’s recent C auris study offers some insights for both medical institutions and the general public.
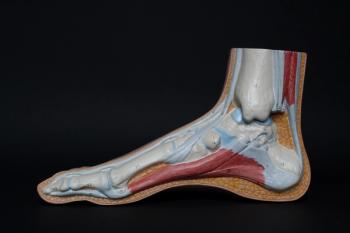
An intensive care physician offers some insights looking at fluoroquinolone-associated tendinopathy and using this social media platform to provide education and an open discussion around it.

On this day of commemoration, The Bill & Melinda Gates Medical Research Institute has multiple TB therapies and a prophylactic vaccine in development, and its Chief Medical Officer Michael W. Dunne, MD, remains optimistic long-term about the state of clinical care, especially in areas of dire need such as low-and-middle-income countries.
Climate change is causing ticks and mosquitos to migrate further north and with them comes disease, previously not seen in these geographic areas. One company is using data to model predictive vector habitats to help organizations and governments identify patterns and help prevent the spread of such diseases.
The Wistar Institute’s Amelia Escolano, PhD, is developing a novel approach using sequential immunization to capture protection against multiple strains.
A young woman who dealt with reoccurring Clostridioides difficile (CDI) in a short amount of time recounts her experience and how getting into a clinical trial made the difference in resolving her condition and getting her back to good health and her family.
Primary care physicians and advanced practice providers are responsible for the majority of people seeking medical care in the United States, yet there is a gap in discussing sexual health for those who are at risk of HIV or other sexually-transmitted diseases.
VisualDx is a platform that can provide clinicians with assistive technology and burgeoning providers with patient education.
This fungal infection is an example of either being a treatable ailment or one that can cause severe disease and death depending on access to diagnostic and treatment resources.

“The biggest take-home message is to treat early,” says remdesivir investigator Mark Thrun, MD.
A new report shows these providers are playing a larger role in vaccine administration, but without federal legislation codifying PREP, half the US states could determine not to continue this path for pharmacists and take away this now vital responsibility.

Remdesivir reduces COVID-19 mortality, in hospitalized patients who both did and did not require oxygen.

Despite effective vaccines and other prevention and treatment modalities, hepatitis B remains a global health challenge. “It’s not for the lack of available tools,” says professor and hepatologist H. Nina Kim, MD, MSc.

“Patients deserve more than just viral suppression,” said Harmony Garges, MD, chief medical officer of ViiV Healthcare.
A researcher who presented at CROI discusses this prolonged and debilitating condition.
A clinician presenting at CROI discusses the phenomenon and offers insights and considerations for treating patients who are dealing with this.

“We should test everybody,” said Charles Béguelin, noting that the vast majority of hepatitis D infections go undetected.

“One size never fits all,” says hepatologist Anna Suk-Fong Lok, MD, emphasizing the need to tailor hepatitis B treatment to fit the patient.

A clinician offers insights on the initial conversations around learning to treat a chronic disease and the importance behind getting patients started down the right treatment path in the early days following a diagnosis.

The Conference on Retroviruses and Opportunistic Infections (CROI) begins today and SARS-CoV-2 has developed into a sustained topic at this annual conference.

A prominent researcher offers some insights on this emerging fungal infection.
Looking to appeal to the financial incentives of antibiotic development, a recent policy paper explores how lives can be saved and a return of investment (ROI) can be realized.

This live-attenuated investigational vaccine was developed by Codagenix, which uses its codon de-optimization platform for its candidates.
Clostridioides difficile (CDI) and recurrence can create a tremendous burden on patients’ quality of life as well as become a financial burden to individual healthcare systems thus creating downstream costs for individual hospitals.

A new Infectious Diseases Society of America (IDSA) board member and vice president offers some insights on the leadership’s goals and direction for 2023.

One clinician offers some insights on the RSV surge witnessed last year and the prospective benefits of having a maternal vaccine to protect newborns from this virus.