Natural infection plus COVID-19 vaccination in pregnant mothers conferred more durable antibody responses in infants than natural infection alone.

Natural infection plus COVID-19 vaccination in pregnant mothers conferred more durable antibody responses in infants than natural infection alone.
The findings may support further COVID-19 vaccination in children and reduce vaccine hesitancy.
The findings emphasize the importance of reviewing all hepatitis B serology reports prior to switching HIV treatment.

The algorithm sought to address high rates of false-positive cultures, excessive antibiotic use, and unnecessary diagnostic procedures in an emergency department setting.
The combination antibiotic was equally effective against both monomicrobial and polymicrobial Acinetobacter baumannii-calcoaceticus Complex infections.
The monoclonal antibody demonstrated a significant impact on RSV-related hospitalizations regardless of infant characteristics.

Adding the oral therapy to standard of care helped reduce total days on oxygen for those hospitalized with COVID-19.
When implemented broadly, malaria vaccines can have a significant impact on public health.
The updated emergency user authorization follows similar approvals and authorizations for Moderna and Pfizer's COVID-19 vaccines.

The expanded indication to those with severe hepatic impairment has already been granted in the US and EU.
Those with certain comorbidities, including peptic ulcer disease and renal failure, were at additional risk of developing C diff infection.

A global review demonstrates the potential depth of the impact of the COVID-19 pandemic on ongoing immunization delay and skepticism.
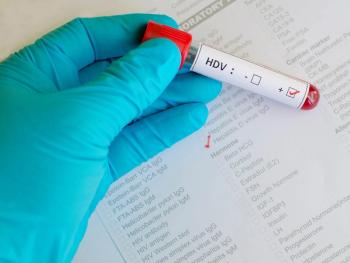
Several trial participants experienced concerning hepatobiliary events.
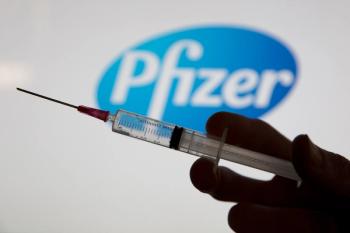
The vaccine is intended to help prevent lower respiratory tract disease caused by respiratory syncytial virus in infants from birth to 6 months of age.
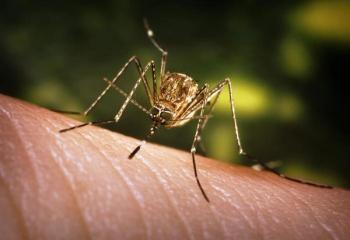
The vaccine is steps behind another Chikungunya vaccine candidate from Valneva, which is currently under priority review by the FDA.
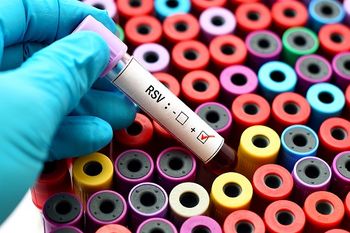
The lawsuit comes as the RSV market is likely to get more crowded, with a pending approval for Pfizer's pediatric indication later this month and a BLA submission from Moderna recently filed.

Marrazzo is currently the director of the Division of Infectious Diseases at the University of Alabama at Birmingham, and will assume her role at the NIAID in the fall.
The vaccine targets an additional 8 unique serotypes that disproportionally affect the adult population.

The investigational vaccine was effective in reducing both lower respiratory tract and all respiratory tract infections.

Distance to the clinic negatively impacted whether the HCV-positive individual attended their first HCV visit.

Published: August 21st 2023 | Updated:

Published: October 19th 2022 | Updated: