
Supported by phase 3 SCORPIO-PEP trial data, ensitrelvir could become the first oral therapy to prevent COVID-19 following exposure, with an FDA decision due June 16, 2026.

Supported by phase 3 SCORPIO-PEP trial data, ensitrelvir could become the first oral therapy to prevent COVID-19 following exposure, with an FDA decision due June 16, 2026.
In the DAN-RSV trial of 131,000 participants, RSVpreF lowered overall cardiorespiratory hospitalizations but showed no significant reduction in cardiovascular events.
Ciprofloxacin monotherapy was non-inferior to aminoglycoside/ciprofloxacin combination for bubonic plague in multi-year trial in endemic region.

Pranita Tamma, MD, MHS, continues her conversation about a study she was involved in that compared ceftolozane-tazobactam and ceftazidime-avibactam. She discusses how clinicians can help reduce the emergence of antimicrobial resistance by using higher doses with extended infusions, limiting treatment duration, ensuring source control, and avoiding unnecessary dose reductions during CRRT.
Results from a Danish trial of more than 330,000 adults 65 years or older found high- and standard-dose influenza vaccines had similar effectiveness against influenza or pneumonia-related hospitalizations.

Robert Hopkins, Jr, MD, medical director of the National Foundation for Infectious Diseases (NFID), discusses what patients are asking about with regards to the vaccines and what the general public should continue to know about their medical value.

This week, expert insights highlighted progress in reducing C difficile with electronic hand hygiene monitoring, advancing HIV prevention with lenacapavir, updating COVID-19 vaccines for high-risk groups, and more.

Spikevax and mNEXSPIKE authorized for older adults and high-risk individuals; experts emphasize protection against severe COVID-19 outcomes.

UNC’s Sarah Rutstein, MD, PhD, discusses her research uncovering the inability of some people to receive HIV prevention medication in both the US and Africa and strategies to address it.

New authorization limits shots to adults ≥65 and individuals with underlying health conditions, with ACIP set to review guidance and insurance coverage implications.

C difficile remains a major healthcare-associated infection. Despite clear guidelines, hand hygiene compliance often falls short, creating gaps between policy and practice. A case study from a hospital showed that after implementing an electronic hand hygiene monitoring system with behavior prompts, compliance improved.
CDC encourages travelers to take enhanced precautions, get vaccinated, and prevent mosquito bites in affected areas, with special warnings for pregnant individuals.

Joseph Eron, MD, provides insights on the unit's international reach as well as some of the important trials it has been involved in, notably the PURPOSE studies involving the landmark trials about lenacapavir for HIV prevention.

UNC’s Benjamin Smith, MD, provides some insights into the logistics and steps behind these complicated and serious missions.

NYC officials confirm 113 cases and six deaths in Central Harlem cooling tower–linked outbreak; separate Bronx investigation underway after cases tied to hot water system.

Infection preventionists face constant regulatory, organizational, and infectious disease challenges, and leveraging evidence-based practices alongside surveillance technology helps them maintain consistency, resilience, and patient safety.

From chikungunya setbacks to COVID-19 booster guidance and childhood vaccine safety initiatives, here are the top regulatory and research developments this summer.

Leaders from the UNC Special Pathogens Response Center discuss how they plan and carry out biopreparedness trainings to address transport and care of patients with high-consequence infectious diseases, such as Ebola or Lassa fever.

Allergist and immunologist Juanita Mora, MD, urges vaccination, masking, and outreach to safeguard vulnerable communities.
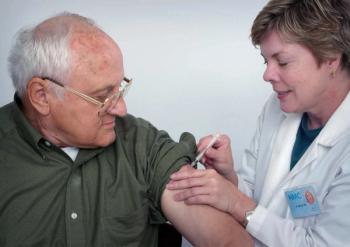
The American College of Cardiology is providing recommendations on the influenza, COVID-19, RSV vaccinations and others.

A 9-month pilot study showed a 55% reduction in resident colonization with MRSA, VRE, and ESBL, but contamination of high-touch surfaces persisted in rooms of MDRO carriers.

Christopher Sellers, MD, discusses its distinct program and diverse professional opportunities and provides some insights as to why many ID trainees stay at the University of North Carolina.
Systematic review finds FMT and standardized microbiome products outperform antibiotics in efficacy and safety.

Regulators cite four new serious adverse event reports, halting US distribution while global access efforts continue.

The NARROWS framework, developed within the IDSA Core Antimicrobial Stewardship Curriculum, provides a structured, behaviorally informed approach to communication that helps stewardship teams address the social, emotional, and cultural factors influencing prescribing decisions in order to optimize antimicrobial use and combat resistance.

This week, people with HIV are living longer on antiretroviral therapy but face elevated comorbidity risks, requiring tailored screening and multidisciplinary care as they age, and more.

New data show consistent late-summer and winter surges, reinforcing the need for seasonal vaccine timing and focused protection for high-risk groups.

The Institute's Director Myron Cohen, MD, discusses the uniqueness of the institution and its ability to offer research and clinical opportunities globally as well as play a significant role in public health.

STOP-PASC substudy results find digital biometrics align with symptom burden, supporting their use as objective measures in long COVID research.

Allegheny Health Network’s (AHN) Thomas Walsh, MD, talks about Allegheny General Hospital being recognized as an Infectious Diseases Society of America (IDSA) Center of Excellence and some of the stewardship strategies they are employing to reduce antimicrobial usage.