
How acute malnutrition undermines Malaria in children under 5 years old.
A large European study looked at a modern-day communication method and its influence on a public health initiative to increase immunizations.
Low-frequency resistant cells in bacterial isolates are challenging to detect and may contribute to unexplained treatment failure.

FDA recalls of specific lots of contaminated products.
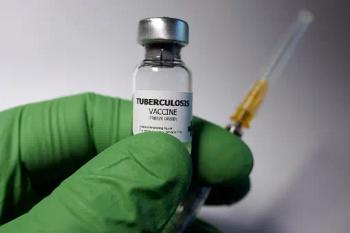
The Bill & Melinda Gates Medical Research Institute launches the M72/AS01E vaccine in South Africa to combat TB.
The company’s investigational vaccine, V116, is a conjugate vaccine and demonstrated immunogenicity for all 21 serotypes across the studied adult groups.

CDC on enhancing strategies against drinking water diseases.

In the latest issue, Editor-in-Chief Jason Gallagher, PharmD, FCCP, FIDP, FIDSA, BCPS, discusses the continuous antibiotic trade-off of targeted vs broad spectrum therapies and cheap vs expensive options as they relate to UTI treatment.
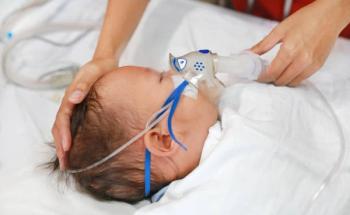
High efficacy of Nirsevimab in preventing RSV hospitalizations was revealed.
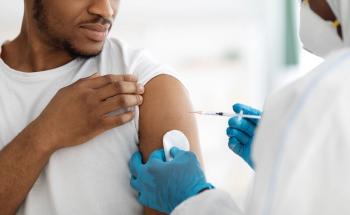
Since changes in vaccine composition can sometimes lead to confusion or misinformation, effective communication is crucial to promote vaccine uptake.

New analysis indicates that children treated at 3 years of age for perinatally acquired HCV will live longer, with less liver morbidity and lower health care costs than if treated at 6 years of age.

Today serves as a reminder to everyone that there are still no diagnostic tests or FDA-approved treatments for the post-viral condition.

A vaccine targeting chronic HBV has entered its first human trial; Saskia Popescu, PhD, on the importance of pandemic preparedness and global health security; a successful phase 2 trial for a C diff treatment leads to the next phase; and more stories this week from Contagion.
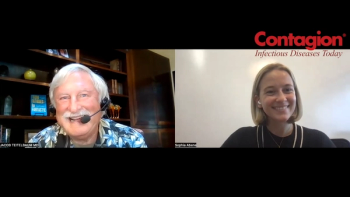
Insights from Jacob Teitelbaum MD on Long COVID Awareness Day.

Saskia Popescu PhD, MPH, MA, FAPIC on understanding the emergence of the origin for future pandemic preparedness, promoting global health security, and addressing long-term health impacts.

The antifungal’s designation ensures its market viability and gives the youngest pediatric patients a therapy for health conditions that have been associated with high mortality rates.

A new study shows how correcting misinformation in a certain way can change patients’ attitudes towards vaccination.
Successful phase 2 trial leads to phase 3 trial towards managing C difficile.
An experienced measles clinician offers insights on the increase of incidence rates, preventative measures, and how vitamin A treatment can help those with the disease.

A new study examined the association of COVID-19 infection with outpatient care utilization.
State health officials did not disclose the origin of transmission.
Vaccine designed to confront chronic HBV entering its first human clinical trial.
It is not simply just a few cases are springing up, but rather that transmission is occurring, and behind a backdrop of vaccine skepticism.

The impact of remdesivir across age groups.
Public health strategy in navigating flu season.
The first-in-class entry inhibitor delivered therapeutic benefits over a long study period for patients with chronic infection.

Customizing treatment strategies for enhanced outcomes and longevity.

Patients hospitalized for suspected community-acquired pneumonia received targeted treatment more quickly with PCR testing than with culture-based diagnostics.
Though a small number of infants received nirsevimab in the analysis, results support existing nirsevimab recommendations to prevent serious RSV disease in infants.

The OASIS platform will help outpatient facilities use prescribing data to improve patient care.