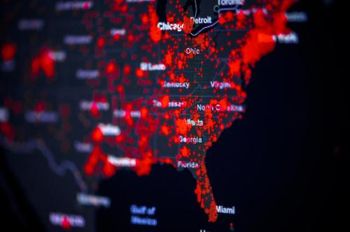
A Harvard Medical School study team sought to identify individuals at elevated risk for severe COVID-19 and, among them, those at high risk of financial ruin as a result of costs associated with their care.

A Harvard Medical School study team sought to identify individuals at elevated risk for severe COVID-19 and, among them, those at high risk of financial ruin as a result of costs associated with their care.

Infectious disease experts have recommended that stockpiles of several promising vaccine candidates be created as they enter late stage development.

Sepsis is characterized by a dysfunctional immune response to infection—norepinephrine can contribute to this immune dysregulation.

COVIDSeq is a qualitative test for the detection of SARS-CoV-2 RNA.

Investigators from the Universitat Autònoma de Barcelona have identified links between antibiotic resistant S maltophilia phenotypes and "quorum sensing."

Patrick Soon-Shiong, MD, describes goals and expectations for the accelerated coronavirus vaccine program Operation Warp Speed.

The test uses whole blood, plasma, or serum. The testing kit is intended to produce reliable results within 10 minutes.

Patrick Soon-Shiong, MD, explains why an ImmunityBio SARS-CoV-2 vaccine candidate uses Nucleocapsid DNA alongside Spike DNA.

Mild symptoms may still present high risk for SARS-CoV-2 transmission.

Due to health concerns, the US FDA is reissuing some EUAs to clarify which respirators are suited for decontamination and reuse.

SARS-CoV-2 arrived in Northern California through a complicated series of introductions internationally and between states.

Clinicians and researchers have questioned the likelihood of assembling the quantity of data supplied by Surgisphere on hydroxychloroquine in such a short period of time.

A brief on the latest infectious disease headlines.

The test measures IL-6 to identify patients with COVID-19 at high risk of intubation.

Antimicrobial stewardship education was conducted with de-identified provider-specific prescribing scorecards.

The trial consisted of non-hospitalized adults who indicated a high-risk or moderate-risk exposure to COVID-19 through the household or an occupational setting.

The vaccine had no serious adverse events reported in the 120 person trial.

John Strotbeck, Olympian and CEO of Boathouse Sports, explains how and why the outerwear company pivoted to PPE production at the start of the COVID-19 pandemic.

A study team investigated the ideal physical distance for avoiding SARS-CoV-2 transmission and assessed the use of eye protection and face coverings in preventing acquisition.

As of June 1, there have been at least 6 cases of Ebola detected in Mbandaka, a port city with a population of more than 1.2 million people.

Cefiderocol functions as a siderophore and has a novel mechanism of action which penetrates the outer cell membrane of Gram-negative pathogens.

A daily infectious disease news update

The FDA has approved bedaquiline for use in combination therapy for the treatment of patients 5 years and older with pulmonary multi-drug resistant tuberculosis.

Preliminary results were highly anticipated, given Anthony Fauci, MD, made optimistic statements about the drug in late April.

The vaccine candidate demonstrated neutralizing antibody and T cell immune response against the novel coronavirus.
Monica V. Mahoney, PharmD, BCPS AQ-ID, BCIDP chats with assistant editor Grant M. Gallagher on the evolving nature of outpatient infectious disease care in light of the COVID-19 pandemic.

Today's trending stories in infectious disease news.
Miriam Isola, PhD, talks to us about the use of phone apps for COVID-19 contact tracing.

In all trial participants to date, the vaccine candidate led to seroconversion with binding antibody levels either at or above levels seen in convalescent sera.

New cases came to the attention of public health officials within Shulan on May 7.