Dr. Rodney E. Rohde, clinical laboratory scientist, discusses campus reopening as well as his work on rabies eradication.

Dr. Rodney E. Rohde, clinical laboratory scientist, discusses campus reopening as well as his work on rabies eradication.
Is it still safe to take your child to get a vaccine during the pandemic? According to experts, there are safety measures in place for this critically important task.

J&J is evaluating both 1- and 2-dose regimens of the adenovirus vector investigational vaccine.

Rezafungin appears safe and efficacious in a comparison with caspofungin. The novel antifungal could facilitate easier treatment courses for patients with candidemia or invasive candidiasis.

Dr. Debra Goff describes the rush to "Just in Case" prescribing of antibiotics early in the COVID-19 pandemic, when less was known about the clinical course of the disease.

Rheumatologists are understandably concerned regarding immune suppressed patients amid the coronavirus (COVID-19) pandemic. What are the risk factors for hospitalization? And do drugs which act against inflammation complicate matters?

On Friday, new guidelines from CDC sought to show how the pandemic coronavirus (COVID-19) spreads. By Monday, those guidelines changed.

Dr. Shervin Molayem, DDS, continues our discussion on his recent paper The Mouth-COVID Connection: Il-6 Levels in Periodontal Disease — Potential Role in COVID-19-Related Respiratory Complications.

Do the norms of media coverage of SARS-CoV-2 worsen rising acute stress and depressive symptoms in the United States?

Antibodies are just one part of the body's immune response. And growing evidence suggests many of us could already have cross-reactive cellular immunity to the “novel” coronavirus.
Dr. Shervin Molayem, DDS, outlines his recent paper The Mouth-COVID Connection: Il-6 Levels in Periodontal Disease — Potential Role in COVID-19-Related Respiratory Complications.

A health data analytics expert describes how repeat or low-value studies can arise amid the rush to publish pandemic research.
An editorial advocating a new method of CRISPR testing was published in NEJM by a team of scientists from MIT, University of Washington, and Brigham & Women’s Hospital.

MediFind's Patrick Howie examines the consolidation of resources during pandemics amid concerns that funding will not return to other areas of health research.

The logistics of supplying the vaccine are being handled by a collaboration of federal agencies. The DoD, parts of HHS, and CDC are coordinating supply, production, and distribution.

Incredible resources and effort have been put into the COVID-19 response, but other infectious disease priorities have also been displaced. Patrick Howie, CEO of a firm which aggregates clinical analysis and data, explains.

Study suggests climate change may shift which areas in the US have optimal temperatures for West Nile virus transmission.

Lockdowns implemented for pandemic control have broad health, social, and economic consequences. This is especially true for people who live and work in slum communities around the world.

University of Maryland professor of medical technology answers where limited testing resources ought to be directed given pandemic supply issues.
Though it may be associated with the past in popular culture, tuberculosis is still the world’s most fatal infectious disease, killing an estimated 1.5 million people each year.

Delayed or avoided medical care can increase death, severity of illness, and the chronic health impacts of acute illness.

Upping the phase 3 investigational vaccine trial size to 44,000 participants will also allow for the enrollment of new populations.

University of Maryland professor of medical technology explains the ways speed and accuracy are weighed when testing is deployed to detect COVID-19.

In another of many hand sanitizer recalls since the start of the pandemic, FDA is working with Medek LLC to remove all lots of “M” hand sanitizer from store shelves.

A quick debrief of the week’s top FDA approvals, FDA authorizations, or other infectious disease pipeline developments from the past week.
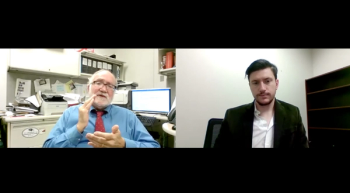
Robert H. Christenson, PhD, University of Maryland, outlines the incentives for how SARS-CoV-2 testing reagents are acquired by institutions.
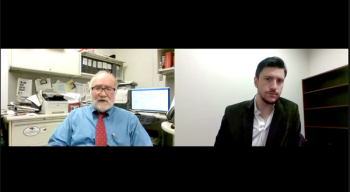
Robert H. Christenson, PhD, outlines the ways SARS-CoV-2 transmission is prevented during testing, how testing materials are acquired, and more.

A new study in The Lancet maps trends in vaccine confidence around the world.

New guidance from the Infectious Diseases Society of America offers timely practice advice for the clinical treatment of three of the most common drug-resistant pathogens.

Decision Diagnostics Corp. has submitted 2 applications with the FDA for a COVID-19 testing technology that the firm claims can identify the virus in about 10 seconds.

Published: October 15th 2019 | Updated:

Published: October 17th 2019 | Updated:

Published: October 21st 2019 | Updated:
Published: October 22nd 2019 | Updated:

Published: October 22nd 2019 | Updated:

Published: October 23rd 2019 | Updated: