
Michael R. Page, PharmD, RPh
Articles by Michael R. Page, PharmD, RPh

Recently, FDA reviewers met to discuss a new drug application submitted by Cempra Pharmaceuticals to treat community-acquired bacterial pneumonia.

The US Food and Drug Administration has approved Selzentry for use in pediatric patients two years of age or older, who weigh at least 10 kg.
Changes in strain prevalence indicate that the existing 9-valent HPV vaccines may offer some protection against an additional high-risk strain that is not included in the vaccine (strain 31), potentially due to antigenic similarities with other strains.

A new CDC study suggests that a closer look at the epidemiology of cat scratch disease may help prevent infections.

The US Food and Drug Administration has approved its quadrivalent formulation of the Flublok influenza vaccine.

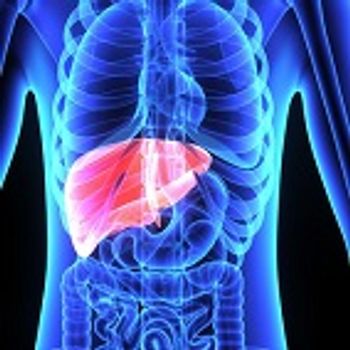
The FDA has released a warning on the risk of reactivation of hepatitis B virus in some patients receiving direct-action antivirals to treat hepatitis C infection.

Researchers have reported the results of an investigation of the role of an immune signaling pathway that may have an important role in mediating the immune response to influenza A virus infection.
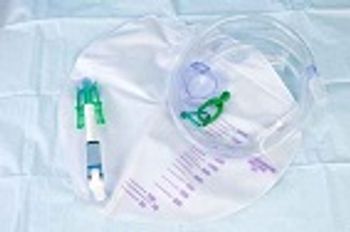
Researchers in the United Kingdom reported results of an in vitro study evaluating a urinary catheter that visibly warns health care providers when an infection is developing.
Seasonal influenza results in nearly 50,000 deaths each year in the United States alone, and 5 to 10 times as many deaths in all industrialized countries combined.

On March 8, 2016, researchers at the Ulsan National Institute of Science and Technology reported the development of an antibacterial fabric that inhibits the growth of bacteria, including antibiotic-resistant bacteria.

In patients with chronic HBV, the immune system may have very low levels of the virus. When reactivation occurs due to immunosuppression, these viruses begin to replicate again. Common symptoms of this event include inflammation of the liver and elevations of liver enzyme levels in the blood. In some cases, bilirubin levels may rise in response to reactivation of infection.

Although some meta-analyses have called into question the efficacy of antiviral treatment, the Centers for Disease Control recommends empirical use of antiviral medications in patients with symptoms suggestive of influenza.
Latest Updated Articles
 An Overview of Infectious Disease Medications Approved in 2016 and Outlook Through 2017
An Overview of Infectious Disease Medications Approved in 2016 and Outlook Through 2017Published: January 2nd 2017 | Updated:
 New Antibiotic Dye May Help Prevent Infectious Diseases
New Antibiotic Dye May Help Prevent Infectious DiseasesPublished: April 4th 2016 | Updated:
 A New Biomarker Test May Help Researchers Develop a More Effective Influenza Vaccine
A New Biomarker Test May Help Researchers Develop a More Effective Influenza VaccinePublished: April 22nd 2016 | Updated:
 Novel Catheter Coating May Provide Early Warning of Infection
Novel Catheter Coating May Provide Early Warning of InfectionPublished: May 24th 2016 | Updated:
 A New Approach to Influenza Therapy: Reducing the Serious Risks of Influenza by Targeting an Inflammatory Pathway
A New Approach to Influenza Therapy: Reducing the Serious Risks of Influenza by Targeting an Inflammatory PathwayPublished: May 25th 2016 | Updated:
 FDA Adds Black Box Warning to Direct-acting Antivirals: Relevance and Guidelines for Managing the New Warning
FDA Adds Black Box Warning to Direct-acting Antivirals: Relevance and Guidelines for Managing the New WarningPublished: October 5th 2016 | Updated: