Selective serotonergic reuptake inhibitors (SSRIs) and beta-adrenergic blockers among agents with potential to mitigate chronic wound infections.

Selective serotonergic reuptake inhibitors (SSRIs) and beta-adrenergic blockers among agents with potential to mitigate chronic wound infections.

CEO Larry D Sutton, MD, PhD, elaborates on the company's strategy and the science behind SIDIPREV to prevent C difficile infections in hospitalized patients.

Rising temperatures and changing humidity are undermining natural pest control, putting North American forests at risk.
In a retrospective cohort study, a health system found this form of prophylaxis led to substantial reductions in 2 of 3 sexually transmitted infections (STIs) in a mostly male population.
Nationwide norovirus cases have recently increased, followed by an FDA warning for consumers to avoid oysters contaminated in a recent outbreak.
The Louisiana Department of Health announced the news late yesterday, and it is the first death in the US associated with the H5N1 virus.

A novel approach may identify resistance earlier and get patients on a proper course of antifungals sooner.

A case series of 13 patients suggests that extended courses of Paxlovid could benefit some Long COVID patients, but the results are inconsistent.
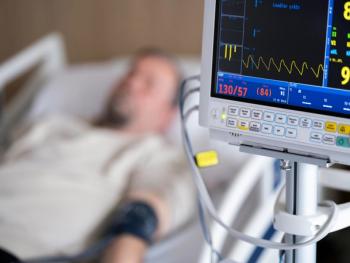
Older males who had these infections had a statistically significant higher risk for physical and cognitive impairments.

Recent study highlights Paxlovid’s ability to reduce hospitalizations and mortality in high-risk populations, but underscores the need for personalized treatment.

Investigators evaluated strategies to simplify the vaccination process to yield higher rates of adult immunization, similar to that of the COVID-19 pandemic.

This week, the CDC MMWR highlights a major blastomycosis outbreak and increased tularemia cases, CHOP's feedback reports improve antibiotic stewardship in pediatric pneumonia, and more.
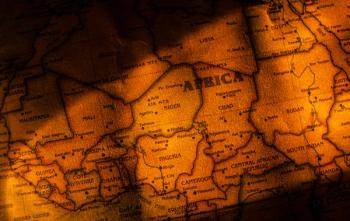
Over a third of respondents in 6 African countries were hesitant to get the vaccine themselves. And for parents, nearly 40% were reluctant to get their children immunized.
The federal health agency stresses the importance of finding the balance between vigilance and enjoying normal activities.
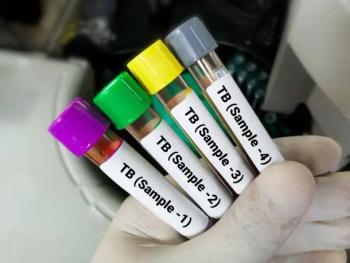
New recommendations emphasize shorter, all-oral regimens for both drug-susceptible and drug-resistant TB.

Just 60% received detectable antibodies for 2 FDA-approved vaccines, possibly pointing out a need for additional doses to reach protective levels against disease.
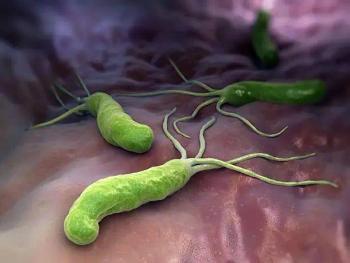
Systematic reviews and localized studies reveal rising resistance rates in pediatric H pylori infections, highlighting the need for improved diagnostics and treatment approaches.

Data detailing antibiotic choice and duration derived from electronic health records improved appropriate therapy use for community-acquired pneumonia.

The largest blastomycosis outbreak linked to a paper mill in Michigan and a 56% increase in tularemia cases over the past decade.

As 2024 winds down, we're looking back on some of the stories that caught readers' eyes on Contagion related to the variety of hepatitis virus infections.

Exploring the challenges and strategies for combating MDR Pseudomonas aeruginosa infections, as discussed with Jason Pogue, PharmD, BCPS, BCIDP

From foodborne illness outbreaks to waterborne diseases and beyond, this year saw developments in the gastrointestinal infection space.

Emerging data reveal surprising patterns in maternal–fetal outcomes linked to avian influenza virus infection.

This week, concerns over mutated avian flu in a Louisiana patient, the potential of ibuzatrelvir for COVID-19 treatment, a comparison of ceftolozane-tazobactam and ceftazidime-avibactam for pneumonia treatment, and more.

Sequencing shows differing virus in the hospitalized patient in Louisiana, which the federal agency says is concerning.

Here are some of the bigger stories on this topic from this year.

A lot of Systane Lubricant Eye Drops Ultra PF has been voluntarily recalled by the manufacturer.

As the year is ending, here are some of the bigger stories Contagion covered on this topic.

As 2024 winds to a close, take a look back on some of the biggest stories Contagion was following around respiratory infections.

Investigators at the University of Virginia are examining the potential link between the “sympathetic” nervous system and the role of the seriousness of the infection.