
Can duration of therapy requirements at the time of antibiotic order entry decrease antibiotic use?

Can duration of therapy requirements at the time of antibiotic order entry decrease antibiotic use?

Raven Boone, DO, discusses her experience in the Temple University fellowship program and what she is exposed to in terms of cases and learning opportunities.

Ep 2, Part 1 of 4: How infectious diseases may fuel global instability in a conversation with psychiatrist Robert Bransfield, MD.


Unsaturated fats in vegetable oils help reduce inflammation and modulate organ metabolism, leading to decreased ALT, AST, and TBIL levels.

In the second part of her interview, Temple’s Stephanie Spivack, MD, talks about how she works with different specialties at the hospital to help this marginalized population receive the care they need.

Global modeling identifies vertical transmission burden and underscores need for maternal screening policies.

In the second part of an interview with Andrea Prinzi, PhD, MPH, SM(ASCP), she talks about how the March of Dimes played such a significant role against the disease, how the polio oral vaccine is a singular lesson that can be applied to medical science as a whole, and the understanding of the need to adapt to changing circumstances.
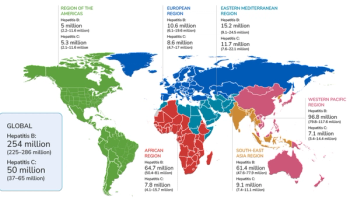
First representative data highlight regional and demographic variations, underscoring the need for enhanced vaccination and targeted interventions.

Andrea Prinzi, PhD, MPH, SM(ASCP), provides insights on how polio cases rose despite sanitation and hygiene improving as well as Jonas Salk’s and Albert Sabin's approaches to their vaccines' development.

This week, AI speeds antibiotic discovery, Temple’s ID team celebrates tradition, EDs boost Hep C detection, thimerosal exits flu shots, and the pandemic’s silent toll on brain health emerges.

A long-acting HIV-1 capsid inhibitor demonstrates over 99% efficacy, paving the way for expanded access to adults and adolescents across the EU and low-income countries.

Stephanie Spivack, MD, talks about the diverse educational experiences afforded to fellows during their time at Temple University as well as the devoted clinical care that serves the local community.
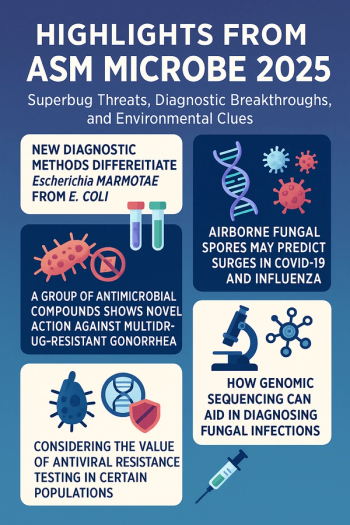
From predicting viral outbreaks via fungal spores and combating drug-resistant gonorrhea to advancing fungal detection through genomic sequencing and improving antiviral resistance testing for vulnerable patients, and more.

César de la Fuente, PhD, provides insights on the promising work of his lab as they accelerate the speed of finding new antimicrobial molecules.

At ASM Microbe 2025, Pelumi Oladipo discusses E marmotae’s reduced motility, misidentification as E coli, and the diagnostic tools closing the gap.
This comes after the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) made the recommendation to remove the vaccine preservative.
New research reveals accelerated brain aging in healthy adults during the COVID-19 pandemic, highlighting broader neurological and social effects.

In the largest world study, there was a 98% reduction in hospitalizations of babies who received the RSV vaccine compared with those who did not receive the RSV vaccine.

Lin Zhu explains why testing alone will not achieve HCV elimination without better access and integrated care.

In the second installment of our Media Day interview, Temple University Hospital’s Rafik Samuel, MD, talks about what he has experienced as a clinician seeing patients with HIV before there were treatments available as well as his approach with fellows in teaching them about this disease.

CDC reports 11 people sick across 10 states with Salmonella linked to frozen sprouted beans causing diarrhea, fever, and severe illness in vulnerable groups.

Rafik Samuel, MD, chief of the Section of Infectious Disease at Temple University Hospital and professor of medicine at Lewis Katz School of Medicine at Temple University, talks about its history and the uniqueness of its fellowship program.

Presented at ASM Microbe, Pelumi Oladipo's study identifies the first North American human case of E marmotae infection and introduces methods to improve species-level diagnosis.

First author Jacek Skarbinski, MD, offers insights on new data published last week showing how this population can benefit from continued vaccination.

The most successful FMT approach involved administering multiple-dose capsules or colonoscopy following an extended course of antibiotic pretreatment.

Shaun Yang, PhD, D(ABMM), FIDSA, MLS(ASCP) talks about how his team developed a test to identify fungi, and how they utilize it to diagnose, make treatment adjustments, and rule out hospital acquired infections.

Jason Haukoos, MD, on how the DETECT Hep C trial leverages 24/7 emergency care to reach vulnerable patients.

Margie Lee, DVM, MS, PhD, wants young researchers and providers to know that despite the current environment, this is not the first time science has had to deal with a challenges to research and funding.

Edward Weinstein, MD of Pfizer, says longer courses may benefit severely immunocompromised patients, even without major gains in viral suppression.