
In this week's episode, the panel discusses therapies and care involving inpatients, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and remdesivir.

In this week's episode, the panel discusses therapies and care involving inpatients, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and remdesivir.
Sexual transmission was confirmed for nine of the 22 viruses studied, including Ebola, Zika, and mpox, making it a key factor in ongoing disease spread.

Jeanne Marrazzo, MD, MPH, director of NIAID, discusses the mutation changes needed to make human-to-human transmission easier as well as infection prevention strategies.

Alba Azola, MD discusses the challenges of diagnosing and managing Long COVID and its overlap with ME/CFS, emphasizing the need for individualized care and ongoing research.

The federal agency stated there were cases of RSV lower respiratory tract infection (LRTI) post-immunization for 2 of the company’s vaccines, mRNA-1345 and mRNA-1365.

Navigating the long road of Long COVID involves more than recovering from an infection; it requires specialized, multifaceted care to address ongoing and often invisible neurological symptoms.

The ongoing outbreak in the Democratic Republic of Congo is affecting mostly very young children, and has investigators searching for answers.
Interim findings from the STOMP trial reveal that tecovirimat does not significantly accelerate lesion resolution or reduce pain in mild to moderate mpox cases.
Study found that VRE colonization is linked to higher mortality, increased need for mechanical ventilation, and renal replacement therapy, while MRSA and C diff colonization showed less impact.

The MeMed Severity test can determine results within 15 minutes through a blood test and aims to offer treatment guidance with machine learning.
If confirmed by the CDC, these cases would increase the national total of human infections to 60, with 58 already reported in various states.

Focused prevention policies and support systems have shown to reduce HCV prevalence in at-risk groups. Addressing factors like IDU, lack of income, and alcohol misuse through targeted education and interventions is essential to mitigate HCV infection rates locally and globally.
Hospitalization rates for RSV in children under five are comparable to pre-pandemic levels, with the highest rates observed in infants aged 0-2 months.

A small phase 2 study evaluated Debiopharm’s investigational antibiotic, afabicin, including its clinical response rate and safety profile of 2-3 weeks of treatment for staphylococcal bone and joint infections (BJI).

A multistate outbreak of E coli O157:H7 infections has been served at catering events, restaurants, and schools.
With some young people still feeling the lingering effects of Long COVID years later, new data shows the value of COVID-19 vaccination to prevent the condition altogether.

This week, a Mayo Clinic study found that removing C diff from gastrointestinal pathogen panels reduced unnecessary treatments, a Salmonella outbreak was linked to cucumbers, and a hepatitis C therapy achieved a 98% sustained virologic response in non-cirrhotic patients, among other developments.

Milad Zandi, PhD, on rising temperatures and the spread of mosquito-borne diseases like dengue, Zika, and chikungunya, increasing the risk of outbreaks in tropical and temperate regions.
Study finds antibiotic selection generally follows CAP guidelines but identifies determinants of frequently longer treatment durations.

Study explores the receptiveness to long-acting antiviral solutions in low- and middle-income countries.

Jones offered his insights for discharging patients as well as follow-up outpatient treatment.

In our latest roundtable series, we discuss COVID-19 treatment management and prevention 4 years after the start of the pandemic.

Most outbreaks were caused by Cryptosporidium, a chlorine-resistant parasite, underscoring the need for stricter safety measures and better facility management.

The complex and often frustrating realities that patients face when dealing with a post-infection condition that remains misunderstood by many continue to unfold.

The 2 companies will examine molnupiravir (Lagevrio) using a different formulation that is not approved in any country globally.
A small study shows that 68% of these patients were given this class of medication, which highlights a disconnect between treatment guidelines and clinical practice.
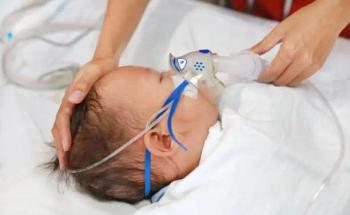
Nirsevimab proves effective in preventing RSV-related medical visits and hospitalizations in infants, with enhanced results in catch-up immunization programs.

The combination therapy’s developer, Atea Pharmaceuticals, is looking to initiate a phase 3 study in early 2025.

Amesh A. Adalja, MD, FIDSA outlined the importance of recognizing antibiotic resistance in healthcare settings, educating patients on responsible antibiotic use, and the need for new treatments and diagnostic tools.

Jacinda Abdul-Mutakabbir, PharmD, MPH, AAHIVP, discusses her work on the Equity in Antimicrobial Stewardship Efforts (EASE) framework, including progress for it, and how other institutions or providers can implement it.