The Omicron variant caused the most symptoms in pediatric patients. However, there were no differences in adverse outcomes by COVID-19 variant.
Nina Cosdon is the associate editor for Contagion. Before joining MJH Life Sciences, she graduated magna cum laude from Denison University in 2021 with a degree in Communication. You can find her reading, hiking, or antiquing, or by emailing her at ncosdon@mjhlifesciences.com.

The Omicron variant caused the most symptoms in pediatric patients. However, there were no differences in adverse outcomes by COVID-19 variant.

There has been a low uptake of bivalent mRNA booster vaccines in older adults, despite a high efficacy of preventing severe and fatal COVID-19.
The innovative antibiotic Xacduro has received FDA approval for its efficacy against ventilator-associated bacterial pneumonia caused by Acinetobacter baumannii-calcoaceticus. The intravenous therapy combines sulbactam and durlobactamto target multidrug-resistant and carbapenem-resistant strains.

Accelerating bivalent booster vaccine campaigns for children could reduce pediatric hospitalizations and school absenteeism.

Today, we celebrate the crucial role of infectious disease pharmacists in combating infectious diseases and promoting patient safety.

Pfizer's RSVpreF vaccine candidate has shown promising efficacy in preventing severe lower respiratory illness caused by RSV in infants. With the potential approval of RSVpreF, pediatric care could undergo a significant positive change.
By implementing rapid point-of-care HCV RNA testing in supervised consumption services, a study achieved high testing acceptance and successful engagement with HCV care among individuals who inject drugs.

Stay informed with this concise summary of the week's most significant stories.

RSVpreF receives strong support from the FDA's VRBPAC, with positive votes for efficacy and safety. The vaccine shows promise in preventing severe respiratory illness in infants, and an FDA authorization decision is expected in August 2023.

These new tests, conducted in a single tube within minutes, could enable at-home testing for various diseases. By incorporating CRISPR technology, the test achieves high reliability by distinguishing between false and true positives.

LMN-201, an oral biologic drug developed by Lumen Bioscience, has received Fast Track Designation from the FDA. The drug combines therapeutic proteins to neutralize the bacterium and toxin that cause C difficile infection.

New findings from clinical trials reveal that Rebyota, a rectally administered treatment, demonstrates improved outcomes in patients with recurrent Clostridioides difficile infection (rCDI).
Empiric therapy for severe Salmonella disease remains effective, but fluoroquinolone resistance calls for a shift towards trimethoprim-sulfamethoxazole as the preferred oral treatment for non-severe cases.

A study of patients with recurrent Clostridioides difficile infection (rCDI) found that Rebyota, a rectally administered live biotherapeutic, was considered easy, quick, and appealing due to the lack of bowel preparation.

This study found a high proportion of reduced vancomycin susceptibility in C difficile patients, leading to lower rates of sustained clinical response.

From the relationship between nutrition and the gut to a newly approved C difficile therapeutic, these are the must-read stories in gastro health.

There are currently no vaccines nor antiviral agents approved for RSV in infants. Sanofi presents data from the HARMONIE trial showing nirsevimab reduces hospitalizations due to RSV by 83.21%.
Antifungal therapy was frequently initiated before essential diagnostic elements confirmed invasive fungal infections in critically ill COVID-19 patients.

Adults who scored higher on the Healthy Eating Index were found to have higher gut microbiota diversity, as well as a higher exercise frequency and lower BMI.

This study determined the efficacy of face masks at reducing exhaled particles in children, as well as whether the type of activity affected the concentration and size of particles.
Recent phase 3 trial data for Vowst showed the microbiota-based therapeutic prevented recurrent C difficile infection in 91.3% of recipients after 8 weeks, a response that 94.6% of these patients maintained through week 24.
Steven Varga, PhD, explains why infants and young children are so susceptible to severe respiratory syncytial virus (RSV) infections.

On the same day WHO Director-General Tedros Adhanom Ghebreyesus declared the end of the COVID-19 public health emergency, CDC Director Rochelle Walensky announced she will be stepping down at the end of June.

Compared to fidaxomicin and vancomycinfecal, fecal microbiota transplant (FMT) was the most cost-effective treatment for first and subsequent recurrent C difficile infection.

RSV expert Steven Varga, PhD, describes the failed vaccine trials that paved the way for the world's first respiratory syncytial virus (RSV) vaccine, as well as the innovations still needed to protect vulnerable populations against RSV infection.

The FDA has authorized GSK’s Arexvy (RSVPreF3 +AS01E) prevent lower respiratory tract disease (LRTD) caused by RSV in adults 60 years and older.

COVID-19 symptom or viral rebound in the absence of antiviral treatment is common, but the combination of symptom and viral relapse is rare.

Decreased racial and ethnic disparities in COVID-19 mortalities between the first and Omicron waves of the pandemic could be explained by increased deaths in non-Hispanic White adults and changes in the geographic spread of the pandemic.
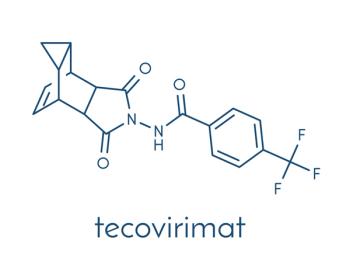
Among mpox patients treated with tecovirimat, there was no difference in treatment outcomes between those living with HIV and those without HIV.
The vaccine efficacy of the Novavax’s NVX-CoV2373 was 79.5% in adolescents.