C. Difficile
Latest News
Latest Videos

CME Content
More News

A European panel of experts endorsed the FDA’s recommendations.
Drs Chopra and Feuerstadt summarize challenges with current treatments and discuss live biotherapeutic products that are being studied for recurrent C difficile infection (rCDI).
Dr Lodise summarizes foundational trials that led to the use of fecal microbiota transplant for the treatment of rCDI.
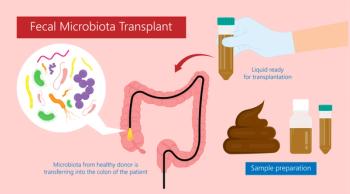
A fecal microbiota transplant (FMT) trial was halted early due to clear evidence the treatment was superior to placebo.

The Vaccines and Related Biological Products Advisory Committee's (VRBPAC) positive vote follows the committee’s review of the data from the biologics license application (BLA) for RBX2660.

Dr Teena Chopra shares her approach to monitoring patients with C difficile infection (CDI).

Drs Tom Lodise and Bincy Abraham provide an overview of fecal microbiota transplantation and long-term safety of FMT use.

Bifidobacterium and Bacteroides in the gut microbiome are important to vaccine responsiveness and could be used in vaccine adjuvants in the future, a recent review shows.
Experts review treatment options for first, second and recurring episodes of Clostridioides difficile infection.

A gastroenterologist reviews long-term impact of dysbiosis and C. difficile infection (CDI).

Dr. Tom Lodise provides an overview of gut microbiota and the consequences of dysbiosis.

Diagnosis and Management of C. difficile Infection (CDI) in Patients with Inflammatory Bowel Disease
Dr. Abraham discusses importance of differential diagnosis of C. difficile infection (CDI) in patients with IBD and how both IBD and CDI should be managed.

A gastroenterologist, Dr. Bincy Abraham, provides an overview of C. difficile infection (CDI) in patients with inflammatory bowel disease (IBD).

Drs. Lodise and Feuerstadt discuss educational opportunities to combat and reduce C. difficile infection (CDI).

Dr. Chopra shares multipronged approach to reducing C. difficile infection (CDI) and the role of antimicrobial stewardship.

Drs. Abraham, Chopra and Feuerstadt discuss importance of timely referral and diagnostic methods to discern C. difficile infection (CDI).

Drs Lodise and Feuerstadt discuss risk factors for C. difficile infection (CDI).

A gastroenterologist, Dr. Paul Feuerstadt, provides an overview of C. difficile infection (CDI) and vegetative and spore phases of CDI.

An FDA advisory committee will meet on September 22, 2022 to decide whether to approve a biologics license application (BLA) for Ferring’s recurrent C difficile biotherapeutic, RBX2660.
Frequent recurrence of Clostridioides difficile infection drives up financial burdens of the disease and underscores the need to improve access to treatment, infection prevention, and new therapies.

Patients with recurrent Clostridioides difficile infection saw improvements in fecal microbiome and bile acid after treatment with the investigational microbiota-based live biotherapeutic RBX2660.

People exposed to a family member recently hospitalized with C difficile infection were 73% more likely to contract CDI themselves.

Patients who took RBX2660 following antibiotic therapy had lower rates of recurrence and double the time to recurrence, compared to patients taking placebo.

The microbiota-based live therapeutic had a 68.3% success rate when administered following antibiotic treatment, versus a 55% success rate in a placebo group.

A clinician discusses the disease burden of reoccurring CDI, but sees a silver lining in the new investigational therapies potentially changing the treatment paradigm for the better.