
HCV / Hepatitis
Latest News

Latest Videos

CME Content
More News

Bluejay Therapeutics’ investigational therapy, brelovitug, offers weekly self-administered injections and will be studied against the only approved treatment outside the US.

This week, people with HIV are living longer on antiretroviral therapy but face elevated comorbidity risks, requiring tailored screening and multidisciplinary care as they age, and more.

A decade-long study finds the ratio independently predicts risk, with patients aged ≥30 facing higher progression rates.

Philippa Easterbrook, MD, MPH, FRCP, explains barriers in primary care, the promise of community models, and how simplified guidelines could expand access to treatment.

Ty Stoner of Walgreens Specialty Pharmacy on navigating barriers, building partnerships, and improving access for vulnerable populations.

Vir initiates phase 2b head-to-head trial of novel combination therapy vs bulevirtide.

This week, from synthetic biosecurity concerns to gaps in maternal hepatitis C screening, rising COVID cases, antimicrobial resistance, fast-tracked CF therapies, and more.
Despite updated guidelines for universal hepatitis C virus screening in pregnancy, testing rates remain well below targets, missing chances to prevent perinatal transmission.

Study reveals notable sex-based disparities in liver complication risk among adults with cirrhosis, particularly in nonviral cases.

The study will examine the switch to the combination of tobevibart and elebsiran in patients not achieving undetectable hepatitis D virus RNA despite bulevirtide treatment.

Unsaturated fats in vegetable oils help reduce inflammation and modulate organ metabolism, leading to decreased ALT, AST, and TBIL levels.

Global modeling identifies vertical transmission burden and underscores need for maternal screening policies.
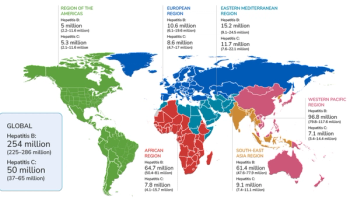
First representative data highlight regional and demographic variations, underscoring the need for enhanced vaccination and targeted interventions.

This week, AI speeds antibiotic discovery, Temple’s ID team celebrates tradition, EDs boost Hep C detection, thimerosal exits flu shots, and the pandemic’s silent toll on brain health emerges.

Lin Zhu explains why testing alone will not achieve HCV elimination without better access and integrated care.

Jason Haukoos, MD, on how the DETECT Hep C trial leverages 24/7 emergency care to reach vulnerable patients.

This week, the first US study screening hepatitis B and D concurrently found that 2.2% of HBV-positive samples had HDV antibodies, the FDA approved GSK’s Shingrix, and more.

Jason Haukoos, MD, MSc, FACEP, shares the decade-long journey behind the DETECT Hep C trial and why emergency departments are key to implementing Centers for Disease Control and Prevention screening guidelines.

Findings From Screening Study for Hepatitis B and Hepatitis D Demonstrate Need for Follow-Up Testing
In our latest podcast, first author Elizabeth Marlowe, PhD, D(ABMM), offers insights on her study looking at this ongoing issue, including the need for increased testing and getting patients into the continuum of care in a more efficient manner.

Study findings underscore the cost-effectiveness of more frequent hepatitis C testing among people who inject drugs, compared to less frequent or no testing at all.
Shortening the OraQuick HCV antibody test from 20 to 5 minutes reduced false positives and unnecessary follow-up RNA testing, but detected fewer viremic individuals.
DETECT Hep C trial shows nontargeted testing in emergency departments outperforms risk-based screening, yet treatment follow-through remains low.

Carl Schmid on the US Supreme Court ruling, proposed Centers for Disease Control and Prevention cuts, stigma, and why equitable PrEP access, including new long-acting options, depends on more than insurance.

HIV+Hepatitis Policy Institute director discusses PrEP access, insurer compliance, and challenges ahead following the landmark decision.

Findings from recent studies published in BMC Gastroenterology highlight practical diagnostic tools and novel therapeutic strategies to improve management of chronic hepatitis B.








































































































































