This market has seen a flurry of activity, as shown by a review of the latest agents.

This market has seen a flurry of activity, as shown by a review of the latest agents.

The patient advocacy organization has events in place and is ready to launch its annual C diff awareness campaign.

Veterans Affairs (VA) study finds some reduction in post-COVID thromboembolic events with early use of nirmatrelvir-ritonavir but no protection against 30 other conditions.

This form of stewardship can reduce unnecessary antibiotic prescribing, reduce costs, and optimize patient outcomes.

Imprecise language can fuel infodemics and misinformation and damage public trust.
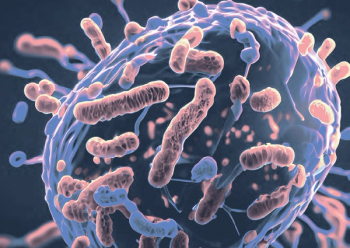
A new study highlights the growing threat of drug-resistant pathogens, particularly in healthcare settings, and underscores the need for enhanced surveillance and intervention strategies to combat these emerging and resistant infectious diseases.

In this week's news, CDC sent out a health advisory to change the prescribing recommendations for the newer preventative respiratory syncytial virus (RSV) monoclonal antibody; a clinician discusses the ongoing challenges presented with critically ill patients and the timing and scope of antibiotic prescribing practices; and antidepressants were associated with a greater risk of Clostridioides difficile infection (CDI).
A comparative study found that using linezolid in place of clindamycin in combination with vancomycin for the treatment of necrotizing soft tissue infections resulted in similar 30-day mortality rates but lower rates of acute kidney injury and composite adverse outcomes, suggesting the effectiveness and safety of linezolid as an alternative therapy.

A new study that went beyond the current standard of testing for the respiratory virus resulted in greater incidence rates and a truer accounting of case loads.

The company dosed its first study participant with its investigational vaccine, mRNA-1083. And this follows recent data reported from their phase 1/2 trial where the vaccine showed strong immunogenicity against influenza and COVID-19, with an acceptable reactogenicity and safety profile.

Emergency departments (EDs) are an important entry point into the health care system and have been identified as a location with opportunity for increased HIV diagnosis and management.

The federal agency sent out a health advisory to change the prescribing recommendations for the newer preventative respiratory syncytial virus (RSV) monoclonal antibody.
This year's Clinical and Laboratory Standards Institute (CLSI) Subcommittee on Antimicrobial Susceptibility Testing provided updated breakpoints on therapies.

Interaction with providers increased testing but did not translate to treatment initiation.
A joint meeting of WHO and FAO offered this assessment of these viruses that millions of people encounter.

If approved, it would become the first nasal flu vaccine to be self-administered.
With increased neutrophils during alcohol-related hepatitis, patients experience an increased risk of infection due to the impaired function of the neutrophils. A new study looks to uncover the mechanisms for which this hepatitis impacts the body.

In the October Editor-in-Chief letter, Jason Gallagher PharmD, FCCP, FIDP, FIDSA, BCPS, writes about this novel antibiotic indicated for a targeted, resistant pathogen and its long-term viability.

This class of medications were associated with a greater risk of Clostridioides difficile infection (CDI).

A clinician discusses the ongoing challenges presented with critically ill patients and the timing and scope of antibiotic prescribing practices.

This week's news included a new study that showed the association with Epstein-Barr virus reactivation and sets the need for an antiviral clinical trial with cardiopulmonary exercise testing as an endpoint; a clinician weighs in on the limited use of novel, gram-negative antibiotics; and the discussion around increasing hepatitis C testing for the pediatric population.

The antiretroviral, lenacapavir (Sunlenca 463.5 mg/1.5 mL injection), had reported resistance in only a minority of study participants according to the latest findings released at a conference.

The Pfizer product is indicated to prevent the 5 most common serogroups causing meningococcal disease in adolescents and young adults.
Resistance against this pathogen with β-lactamase medications can vary according to geographic region.

A study looked at patients’ microbiome profiles to see if they differed at study entry and whether microbiome recovery was dissimilar between subgroups, post-treatment.

Association with Epstein-Barr virus reactivation sets the need for an antiviral clinical trial with cardiopulmonary exercise testing as an endpoint.

Despite hepatitis C (HCV) incidence rates increasing, adequate screening for the virus continues to be a pressing challenge for public health officials.

A study reported at IDWeek looked at this population and found gaps in testing.
Company plans to start recruiting for its REPREVE study this year.

The companies' initial focus will be around Assembly’s established areas of development including hepatitis B and D, as well as herpesviruses.