
Jill Morgan, RN, BSN, talks about steps being taken to ensure everyone’s safety is front of mind.

Jill Morgan, RN, BSN, talks about steps being taken to ensure everyone’s safety is front of mind.

CDC surveillance shows sharp week-over-week increases in influenza test positivity, outpatient illness, and hospital admissions, with A(H3N2) continuing to predominate nationwide.

The recent CDC ACIP meetings and subsequent actions show a path for which the current Health and Human Services department wants to roll back access to vaccines for this virus and other vaccines.

Jill Morgan, RN, BSN, reviews this significant part of clinical care and how personal protective equipment (PPE) is essential to protecting healthcare workers.

Emory's Gavin Harris, MD, discusses these elements, and how despite the fact they rarely occur, the need to do continuous training is essential for readiness.
Shortening course of empiric broad spectrum antibiotics for community-onset sepsis was associated with fewer days of antibiotics and hospitalization and no increase in mortality.

This week, check out our Media Day coverage from Emory, the WHO report on malaria, and more.

In the final installment of the series, psychiatrist Robert C Bransfield, MD, outlines how interdisciplinary research, education, and policy could help reduce infection-associated neuropsychiatric impairment and violence.

In the second installment of this 2-part article on the WHO 2025 World Malaria Report, the authors point out the positive trends in the scaling up of prevention efforts with new-generation anti-mosquito netting, vaccines and periodic chemoprevention.

The WHO 2025 World Malaria Report adds several countries to those certified as malaria free, while recognizing spread of antimalarial drug resistance.

Amy Colson, MD, MPH, discusses phase 2 data showing durable virologic suppression, low resistance risk, and favorable safety with weekly oral islatravir plus lenacapavir in suppressed adults with HIV.

Gavin Harris, MD, discusses his experience working in outbreak environments where there is high-consequence infectious disease (HCID) and how it is important to establish buy-in from the local populations and some of the important elements of what you need to leave behind when an outbreak is declared over.
Agreement supports commercialization and reimbursement efforts for tobevibart plus elebsiran as the ECLIPSE clinical program advances, including completion of enrollment for the ECLIPSE 3 trial.

The single-source award will fund a large randomized trial evaluating mortality, morbidity, and developmental outcomes following the hepatitis B birth dose, amid ongoing debate over non-specific vaccine effects.

This week, learn more about Emory's approach around treating high-consequence infectious diseases such as Ebola, a UNC researcher's work in sequencing syphilis genomes in the search to develop a global vaccine, how the US is in danger of losing its elimination status for measles, and more.

Emory's Gavin Harris, MD, talks about taking care of patients with high-consequence infectious diseases, and considerations for not only their care, but their families. He also discusses working with other healthcare facilities around COVID-19 care and educating them through Emory's ECHO program on new and existing threats.

Zandraetta Tims-Cook, MD, MPH, AAHIVS, discusses access challenges, missed-dose management, and scalable care models for cabotegravir PrEP in women.
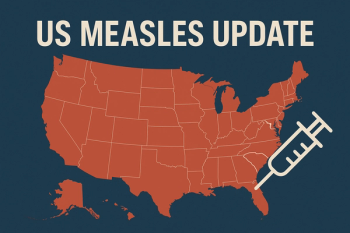
CDC reports over 1,900 confirmed cases and 49 outbreaks nationwide, with recent surges reported in South Carolina, Utah, Arizona, and Connecticut amid declining childhood vaccination coverage.

Jonathan Parr, MD, MPH, discusses his work in locating potential targets to develop a syphilis vaccine and the challenges associated with genomic diversity of subpopulations of the main pathogen, Treponema pallidum.

A Nature Microbiology study shows that structure-guided bile acid design can lock Clostridioides difficile toxin B into an inactive conformation, protecting mice from disease without disrupting the gut microbiome.

This new recommendation relates to infants born to women who test negative for hepatitis B. This is a departure from the federal agency’s previous recommendation and comes on the heels of the ACIP’s recent meeting.

Andrew Handel, MD, talks about transmission, differential diagnosis, treatment, and protection against it.
An impending deadline is coming up in early 2026 that could cause the country to lose its status. However, this can be reversible and unnecessary infections, severe disease, and deaths can be avoided. Rodney Rohde, PhD, talks about incidence rates, how we got here, and strategies to increase immunizations.

Margaret Aldrich, MD, provides insight around this concept, addressing stewardship within this patient population, and how their institution’s collaborative work environment helps all of the department’s clinicians.

Gilead Sciences reported positive phase 3 ARTISTRY-2 results demonstrating that a once-daily single-tablet combination of bictegravir and lenacapavir is statistically noninferior to bictegravir/emtricitabine/tenofovir alafenamide tablets in virologically suppressed adults with HIV. The company plans to file for regulatory submissions for approval.

With a novel, non–cross-resistant mechanism and phase 3 data showing noninferior efficacy to injectable standard therapy, zoliflodacin could become a novel treatment that expands clinician choice and strengthens global efforts to combat antimicrobial-resistant gonorrhea. Innoviva CMO David Altarac, MD, offers further insights about the newly-approved antibiotic and its potential place in the market.

This week, check out our coverage on 2 antibiotic FDA approvals, and clinicians weigh in on the ACIP recommendations on the hepatitis B virus (HBV) vaccine.

The antibiotic's approval was based from phase 3 results demonstrating noninferiority to a combination therapy.

Infectious disease pediatrician Sharon Nachman, MD, talks about how the new recommendations will defer immunization, leading to a whole host of issues and negative impacts on long-term public health.

GSK’s David Payne, PhD, provides more information are the data from the EAGLE-1 trial.