Invivyd’s investigational pemivibart (Pemgarda), which received an FDA emergency use authorization earlier this year, continued to show efficacy in preventing disease onset over a 6-month exploratory period in this patient population.

Invivyd’s investigational pemivibart (Pemgarda), which received an FDA emergency use authorization earlier this year, continued to show efficacy in preventing disease onset over a 6-month exploratory period in this patient population.

The town of Plymouth decided to prohibit people from using the parks from dusk to dawn due to concerns over eastern equine encephalitis after a man contracted the rare disease in the state.
The Phase 3 CLOVER trial still showed safety and potential benefits by reducing C difficile infection duration, medical attention needs, and antibiotic use.
Two recent CDC Morbidity and Mortality Weekly Report (MMWR) studies show stable overall vaccination rates for 2023 but a decline in HPV vaccination rates for certain birth cohorts.

In the second part of our interview with Christian Lillis, cofounder and CEO of the Peggy Lillis Foundation, he discusses what the passage of the Peggy Lillis Clostridioides difficile Inclusion Act would do for reporting of the infection as well as the potential ancillary effects around antibiotic development and antimicrobial resistance.

Nestlé aims to increase the global availability of Vowst, an FDA-approved therapy indicated for a healthcare-associated infection, while also seeking new opportunities for the product worldwide.
In this case study, clinicians review a challenging case.
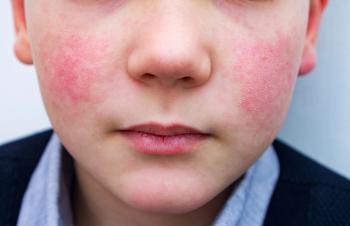
This virus is known for causing mild illness and the distinctive "slapped cheek" rash in children and has recently shown an unusual increase in activity.

Clinicians provide an overview of intraamniotic infections and offer treatment strategies for these challenging infections.

This week, Pfizer-BioNTech's Phase 3 trial of a COVID-19 and influenza combination vaccine yielded mixed results, mpox as a global health emergency emphasizes need for coordinated international response, ongoing challenges with MRSA infections, and more
Understanding the potential and limitations of new Acinetobacter baumannii active therapies.
The study found that vancomycin is more effective than metronidazole in achieving event-free survival for C difficile infections across all infection severity levels.

Transmission to humans is limited in the US, but concerns linger, especially in the wake of the COVID-19 pandemic.

The 2024-2025 season introduces a new vaccine formula with a monovalent component designed to enhance protection against current variants and reduce severe outcomes such as hospitalization and death.

In our latest roundtable series, we cover different aspects of HIV care. In this series, clinicians discuss diagnosis, therapies such as long-acting injectables, treating patients with multidrug resistance and more.

A focus group study reviewed provider practices to learn more about patient management and explore the potential to follow up with clinicians on important takeaways.

The NIH’s RECOVER Initiative has developed new symptom indices for Long COVID in school-age children and adolescents to find 14 symptoms overlapping between the two groups.
This study provides evidence that using DAAs reduces liver fibrosis and improves clinical outcomes.

In the current state of the mpox outbreak, individuals with advanced HIV face a higher risk of severe illness, making vaccination and targeted public health measures essential.

The results indicate that paternal hepatitis B virus infection before pregnancy is linked to a 40% higher risk of congenital heart diseases in their children.
The study found that the vaccine, combined with presumptive antimalarial treatment, reduced Plasmodium falciparum parasitaemia and clinical malaria over two transmission seasons.

Bavarian Nordic is working to get their MVA-BN (Jynneos) mpox vaccine to those affected in African countries, as well as working towards a clinical trial for the younger pediatric population, and potential regulatory approval in Europe for adolescents.

Mpox virus evades the innate immune system by interfering with antiviral pathways signaling and interferon responses, increasing replication, and complicating outbreak management, especially in non-endemic regions.

These skills are as critical as ever, and younger clinicians and learners need them to communicate and work professionally.
Recent study establishes that the generic glecaprevir/pibrentasvir is bioequivalent to the brand name version, offering the same therapeutic benefits.
Despite the differences in clinical presentation, MRSA strains remain susceptible to several key antibiotics, emphasizing the importance of targeted treatment and postoperative education in preventing MRSA infections.
We have complied some of our news stories and interviews from the last week to offer information on the differences between clade I and clade 2 mpox, why WHO declaring the global emergency is significant, looking at mpox in the United States, and more.

The Africa CDC's declaration of the mpox outbreak as a Public Health Emergency of Continental Security, GIGA-2339's upcoming phase 1 trials for HBV, ActivePure Medical's air decontamination system's success in reducing MRSA and HAIs, and more this week from Contagion.

NOWDiagnostics' represents a new option for early syphilis detection and aligns with ongoing efforts to improve STI screening.

The companies’ study met 1 of their 2 primary immunogenicity objectives.