
Under its final guidance, all prospective blood donors will answer a series of individual, risk-based questions to determine eligibility.

Under its final guidance, all prospective blood donors will answer a series of individual, risk-based questions to determine eligibility.

ViiV Healthcare is working with 3 companies to manufacture generic versions of cabotegravir long-acting (LA).

If FDA approved, this 5-in-1 investigational candidate could provide the broadest meningococcal serogroup coverage.
Investigators see a potential new approach to therapeutic development for C diff.

The Lucira COVID-19 & Flu Test is a single-use at-home test kit that provides results from self-collected nasal swab samples in roughly 30 minutes.

In doing so, the country met the new target of the World Health Organization.
The agency would like to begin using individual risk-based questions to reduce the risk of transfusion-transmitted HIV.
The therapies for Clostridioides difficile infection (CDI) saw many developments in recent months and there are more exciting things to come.

They developed clinical guidance and other resources looking to address potential drug interactions with these patient populations who have COVID-19 and may be treated with Paxlovid.
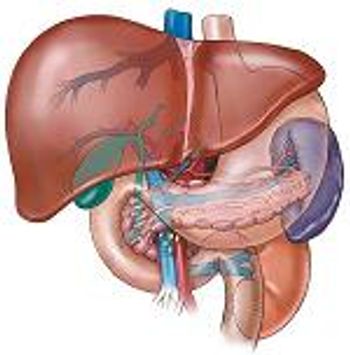
Health programs and efforts to inform the public about the importance of sharing liver education can play a major role in prevention as well as patient care.

A central portal to support clinical practice for patients with or at risk for HIV was launched.

Catch up on all the important news and clinical care topics you may have missed this week.


The revised Emergency Use Authorization (EUA) for Paxlovid (nirmatrelvir and ritonavir) allows these providers to offer this COVID-19 therapy.
This past week has been extremely busy in infectious disease with a lot of vaccine news that could impact young children. In addition, GSK reported on its phase 3 study on its RSV vaccine for seniors.

The FDA VRBPAC votes to recommend mRNA vaccines in youngest children; WHO moves to rename monkeypox; Anthony Fauci is diagnosed with COVID-19; and Pfizer stops recruiting for a Paxlovid trial.

GSK’s Priorix MMR vaccine received the federal agency’s approval which was based from data of 6 clinical trials evaluating its safety.

The White House coronavirus response coordinator said vaccines for children under 5 could potentially begin on June 21.

This is the first test authorized for all 3 viruses and is over the counter.

Here is a rundown of the most popular stories we covered this past week.
Here is a rundown of the most popular stories we covered this past week.
Check out the important stories we covered this week.

The company’s filing for its NVX-CoV2373 vaccine is based off data from 2 large clinical trials.

Pfizer CEO Albert Bourla made the announcement in an interview.

The recall involves 2 lots of 100 mg of remdesivir for injection to the user level.

The agency said the 2 monoclonal antibodies could be used to treat mild to moderate COVID-19 in all younger pediatric patients, including newborns.

Highlights from the sessions of the World Anti-Microbial Resistance Congress 2021 included addressing the global threat for AMR and looking at real world data and real world evidence to deter resistance.

The therapy can be used for the treatment of invasive candidiasis.

The organization is hosting its 9th Annual International C Diff Conference and Health EXPO on Thursday and Friday this week. The conference is virtual and free to the public.

The company announced the Emergency Use Authorization (EUA) for use with its Spectrum Solutions SpectrumDNA SDNA-1000 collection device on the Amplitude Solution.