
A large retrospective cohort study demonstrates greater resistance across different pathogens in this patient population compared with those who do not have cancer.
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

A large retrospective cohort study demonstrates greater resistance across different pathogens in this patient population compared with those who do not have cancer.

Paul Offit, MD, offers a glimpse of when the outbreak might end as well as where we are with herd immunity, the MMR vaccine’s efficacy, and insights on breakthrough cases.

Anu Osinusi, MD provides insights on the findings of Gilead’s investigational therapy from its phase 3 MYR301 study, which are being reported at the ongoing European Association for the Study of the Liver (EASL) Congress 2025.

Anu Osinusi, MD, discusses the findings examining bulevirtide as a standalone therapy and in combination with pegylated interferon alpha.

Atea Pharmaceuticals' investigational combination treatment of bemnifosbuvir and ruzasvir showed these results after an 8-week treatment regimen.

Paul Offit, MD, offers insights on the state of measles today where some families are opting to believe treatment is a better option than vaccination. He also discusses the potential toxicity of vitamin A treatment, as well as the limitations of supportive treatment for the disease.

In a large study, Moderna’s investigational vaccine, mRNA-1083, was shown to have noninferiority to other vaccines, and had an acceptable tolerability and safety profile.

Sean Nguyen PharmD, BCIDP, medical director Shionogi, delves into the European cohort portion of the study and offers further information from the trial and some of its bigger clinical ramifications.

The National Foundation for Infectious Diseases (NFID) released its 2025 NFID State of Handwashing Report and found that many US adults forget or choose not to wash their hands at key times when germs can easily spread.

The company had requested approval for its mRNA-1083 combination vaccine last year. With this setback, the company is now looking at 2026 for approval.

Six states hold the highest percentage of A grades.

The Hecolin hepatitis E virus vaccine was delivered with a reduced 2-dose schedule to people living in a refugee camp to try to reduce an outbreak.

The agencies are calling for sustained investments in immunization efforts in the midst of increasing case numbers rising for diseases such as whooping cough, measles, and others.

A preclinical study shows the antibiotic, piperacillin, was effective at much lower dosages than doxycycline, and did so without disrupting the gut microbiome.

Matthew Hepburn, MD, offers some insights on his time as vaccine development lead for Operation Warp Speed and the ability to develop a COVID-19 vaccine within a year.

The Johns Hopkins Malaria Research Institute’s work in this area is looking to find ways to identify, for the first time, every single gene in the genome of malaria parasites, which could lead to the development of new treatments or vaccines.

Atea Pharmaceuticals will present the full clinical study at the upcoming EASL Congress in May.

The Johns Hopkins Malaria Research Institute is hosting its World Malaria Day Symposium on Friday. Jane M. Carlton, PhD, offers some insights on the institute and its signature event.

Scynexis developed its investigational fungal compound, SCY-247, which is in phase 1 clinical trials, and the company expects to share top-line data this year.

A Centers for Disease Control and Prevention study looked at the COVID-19 pandemic's effect on hospital-onset infections. Study lead author Hannah Wolford offers insights on it, including what patient populations were most affected, and the significance of infection prevention strategies.

Universal hepatitis C screening updates have influenced testing numbers, and resulted in a gradual increase for all women with a pronounced increase in pregnant women.

Matthew Hepburn, MD, executive vice president of research and development at Panther Life Sciences, discusses the technology and its potential usage.
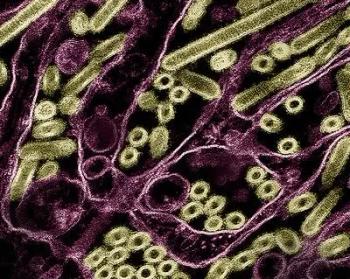
This case represents the second reported human infection with avian influenza A(H5) in Mexico, and the first confirmed case of influenza A(H5N1) in the country. The patient was a child who died from respiratory complications.

A large study showed these SARS-CoV-2 positive groups were more prone to myocarditis, heart failure, arrhythmias, and chest pain—regardless of prior cardiovascular history or presence of congenital heart disease (CHD).

The Centers for Disease Control and Prevention (CDC) says there are now 7 outbreaks.

Precision BioSciences received the designation for its gene editing therapy, PBGENE-HBV, developed to eliminate the root cause of chronic hepatitis B virus (HBV).

In a phase 3 study, GSK’s gepotidacin demonstrated 92% efficacy against the sexually transmitted infection.

Carl Schmid, executive director, HIV+Hepatitis Policy Institute, speaks to these closings.
Although there have been decreases in newly reported chronic hepatitis C cases across many demographics, the need to get people screened and given therapy in the same visit remains vital.

Study shows benefit using antibiotic earlier in clinical care for gram-negative infections.