
CDC surveillance shows sharp week-over-week increases in influenza test positivity, outpatient illness, and hospital admissions, with A(H3N2) continuing to predominate nationwide.
Abene is currently a freelance writer and editor who contributes to Contagion. She is the former Assistant Editor for Contagion. She can be emailed at: sophiaabene@gmail.com.

CDC surveillance shows sharp week-over-week increases in influenza test positivity, outpatient illness, and hospital admissions, with A(H3N2) continuing to predominate nationwide.

In the final installment of the series, psychiatrist Robert C Bransfield, MD, outlines how interdisciplinary research, education, and policy could help reduce infection-associated neuropsychiatric impairment and violence.

Amy Colson, MD, MPH, discusses phase 2 data showing durable virologic suppression, low resistance risk, and favorable safety with weekly oral islatravir plus lenacapavir in suppressed adults with HIV.
Agreement supports commercialization and reimbursement efforts for tobevibart plus elebsiran as the ECLIPSE clinical program advances, including completion of enrollment for the ECLIPSE 3 trial.

The single-source award will fund a large randomized trial evaluating mortality, morbidity, and developmental outcomes following the hepatitis B birth dose, amid ongoing debate over non-specific vaccine effects.

Zandraetta Tims-Cook, MD, MPH, AAHIVS, discusses access challenges, missed-dose management, and scalable care models for cabotegravir PrEP in women.
CDC reports over 1,900 confirmed cases and 49 outbreaks nationwide, with recent surges reported in South Carolina, Utah, Arizona, and Connecticut amid declining childhood vaccination coverage.

A Nature Microbiology study shows that structure-guided bile acid design can lock Clostridioides difficile toxin B into an inactive conformation, protecting mice from disease without disrupting the gut microbiome.

Questions raised about unverified FDA claims linking COVID-19 vaccines to pediatric deaths, and concerns that proposed vaccine testing requirements could delay access to key immunizations.
A large meta-analysis indicates opioid exposure may disrupt immunity and gut microbiota, increasing CDI vulnerability and potentially worsening clinical outcomes.

A public health and farmworker coalition says agricultural spraying of medically important antibiotics and antifungals contributes to environmental resistance and warrants EPA cancellation.
New Centers for Disease Control and Prevention (CDC) data show rising early-season influenza activity, moderate 2024–2025 vaccine effectiveness, and updated 2025–2026 recommendations that include trivalent, thimerosal-free single-dose formulations and expanded access to FluMist.

WHO and UNAIDS warn of service disruptions, widening inequities, and mounting drug-resistance threats, while highlighting innovations and commitments needed to end AIDS as a public health threat.
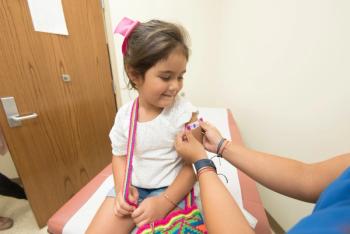
James Conway, MD, describes how full vs partial repeal of nonmedical school-entry exemptions is associated with kindergarten vaccination and exemption trends.

A DOXYVAC substudy found higher rates of tetM-mediated tetracycline resistance and more isolates with decreased cefixime susceptibility among MSM using doxycycline PEP.

Large international study reports higher A-strain protection and acceptable safety profile for quadrivalent RNA influenza vaccine.

David Cameron, PhD, discusses how researchers discovered its antibiotic, Debio 1453, through structure-guided design and advanced it from potent preclinical activity to first-in-human testing.

Primary completion is expected in Q4 2026 with topline results in Q1 2027, while ECLIPSE 2 and 3 continue enrolling to support potential US and EU submissions.

ByHeart Whole Nutrition Infant Formula is the product involved in the recall. Fifteen infants have been hospitalized in 12 states prompting a federal investigation. Clinicians are urged to treat on suspicion and report all cases.

The tool's developer, LIV Process, says it has created a water-based reagent that illuminates Clostridioides difficile on surfaces so teams can verify cleaning in real time.

IND clearance enables a randomized, double-blind study of IMM-529 with standard care, while a forthcoming USU Travelan field readout will guide dosing strategy.

In a multinational NEJM trial, a single 105-mg dose cut RSV hospitalizations by 84.2% and medically attended lower respiratory infection by 60.4% through 150 days, with safety similar to placebo.

Muhammad Sohaib Asghar, MBBS, MD, discusses why CDI deaths are concentrated in White patients and inpatient/long-term care settings, why mortality peaked in 2006–2015, and how stewardship and recurrence prevention are driving the recent decline.
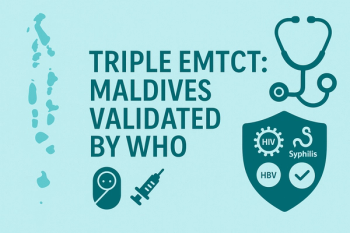
Sustained HIV/syphilis elimination of mother-to-child transmission (EMTCT) since 2019 and new hepatitis B validation reflect high antenatal screening, >95% HBV birth-dose coverage, and integrated maternal–child health services.

Ed J Kuijper, MD, PhD details who is at highest risk for rCDI, how to interpret NAAT–toxin results, and how to sequence fidaxomicin, vancomycin, and microbiota-based therapies.

NIH funds an effort to adapt an electrochemical biosensor for same-visit detection and viral-load quantification at the point of care.

A UK hepatology review found that one-third of adults with chronic hepatitis B were lost to specialist follow-up—highlighting major gaps in patient retention that threaten progress toward WHO’s 2030 viral hepatitis elimination goals.

John Osiecki, PhD, explains how faster, standardized testing can speed detection and strengthen antimicrobial stewardship.

Through the bipartisan Cure Hepatitis C Act of 2025, legislation seeks to provide nationwide hepatitis C treatment and strengthen public health infrastructure by expanding testing, treatment, and prevention through a subscription-based model.

Multicenter study will measure efficacy, safety, and immunogenicity in adults 18–59 across Europe.

Published: April 4th 2025 | Updated:

Published: September 9th 2024 | Updated:
Published: August 5th 2025 | Updated:

Published: April 11th 2025 | Updated:
Published: August 6th 2025 | Updated:

Published: June 27th 2024 | Updated: