
The country successfully managed thousands of mild cases among foreign construction workers at the height of the pandemic through an isolation facility. It may not be an applicable model, though.

The country successfully managed thousands of mild cases among foreign construction workers at the height of the pandemic through an isolation facility. It may not be an applicable model, though.
A pediatric cardiologist discusses the specifics of a case study, the importance of adaptability, and how her healthcare system has developed treatment protocols in these cases.

Medical science needs to understand the disease more clearly in this understudied patient population.
What the AstraZeneca vaccine research pause meant for physician-patient discussion on vaccine candidate safety.

Do the norms of media coverage of SARS-CoV-2 worsen rising acute stress and depressive symptoms in the United States?

The guidance clarification published today comes just a day after reports a polarizing guidance was published last month without full review nor consensus from the agency.

Antibodies are just one part of the body's immune response. And growing evidence suggests many of us could already have cross-reactive cellular immunity to the “novel” coronavirus.
Dr. Shervin Molayem, DDS, outlines his recent paper The Mouth-COVID Connection: Il-6 Levels in Periodontal Disease — Potential Role in COVID-19-Related Respiratory Complications.
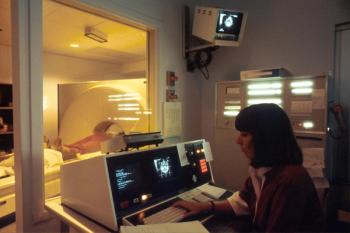
Scans typically used to diagnose strokes are showing physical signs in the lungs that identify COVID-19.
A new study adds to growing concern about short immunity duration and the potential for reinfection.

Officials state the controversial August guidance was influenced by other federal agencies, and that revisions may come soon.

A health data analytics expert describes how repeat or low-value studies can arise amid the rush to publish pandemic research.
An editorial advocating a new method of CRISPR testing was published in NEJM by a team of scientists from MIT, University of Washington, and Brigham & Women’s Hospital.

MediFind's Patrick Howie examines the consolidation of resources during pandemics amid concerns that funding will not return to other areas of health research.
A look into the long-term implications of the virus on athletes' cardiovascular fitness.
What we've seen non-immunized communities do for previously eradicated outbreaks.
Eli Lilly’s investigational therapy, LY-CoV555, was shown to be well-tolerated and met its primary endpoint for one of its doses.

The logistics of supplying the vaccine are being handled by a collaboration of federal agencies. The DoD, parts of HHS, and CDC are coordinating supply, production, and distribution.

Incredible resources and effort have been put into the COVID-19 response, but other infectious disease priorities have also been displaced. Patrick Howie, CEO of a firm which aggregates clinical analysis and data, explains.
A survey from The Ohio State University shows a majority of Americans would get an approved vaccine.
COVID-19 patients require supervised pulmonary rehabilitation to recover ERS researchers say.

Lockdowns implemented for pandemic control have broad health, social, and economic consequences. This is especially true for people who live and work in slum communities around the world.
University of Maryland professor of medical technology answers where limited testing resources ought to be directed given pandemic supply issues.
Though it may be associated with the past in popular culture, tuberculosis is still the world’s most fatal infectious disease, killing an estimated 1.5 million people each year.

In a newly published paper, investigators believe the novel virus will follow similar patterns to seasonal viruses like influenza.