A similar shutdown seems inevitable, but there may be an alternative.

A similar shutdown seems inevitable, but there may be an alternative.

The test simultaneously recognizes the S1 and S2 subunits of the virus' spike protein in one sample, providing better sensitivity.
Neurological symptoms of COVID-19 ranging from dizziness to microclots could be the result of destabilization of the blood-brain barrier caused by virus spike proteins circulating in the blood.

The country moves into a difficult phase of the pandemic as fall slides into winter with no national mandates and no vaccine.
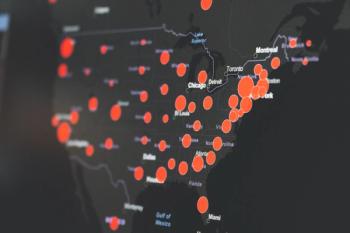
In a survey, a panel of experts sees the American response as problematic and weighs in on universal masking.


Disagreement among health officials, politicians, and the media leads to inconsistent levels of compliance with guidelines.
LY-CoV555 was derived from a patient with COVID-19 and developed by Eli Lilly.

The company recently announced it was in a phase 1 trial.

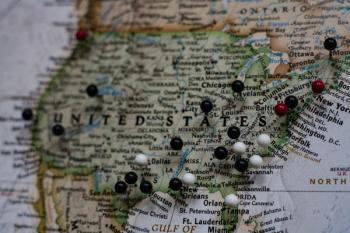
Data suggest New York City COVID-19 cases could have been reduced by 80% in two months if social distancing was implemented a week earlier than it was.
We asked a simple question to our IDWeek experts: has COVID-19 taken attention away from any greater infectious disease issues?
A Medicare program provided more point-of-care testing devices this summer. Yet, even hot-spot facilities are far more likely to receive results in ≥3 days than 1.

Virologists have identified a gene encoded by SARS-CoV-2 that enables it, but not SARS-CoV or Influenza A viruses, to inhibit interferon-based immune response

The investigational antibody used to treat President Trump shows it reduced viral load and medical visits.

A new study shows disproportionate impact of the virus to this group.

As researchers develop ways to assess the effects of the pandemic on criminal activity, a new report documents the impact on those incarcerated.
These vital cells that are recovered from COVID-19 patients might be able to help people with compromised immune systems due to cancer therapy or transplantation.
While this trial for hospitalized COVID-19 patients has been discontinued, Lilly is continuing their other trials with the medication for COVID-19 treatment.

A research team recently focused on how the pandemic intensifies the effects felt by those already marginalized or vulnerable, as it is the intersection of biological and socioecological factors.

The company is planning to move forward after receiving a recommendation from the DSMB to start up the trial again.
A case study examines a treatment for a hospitalized case of the virus.

Autoantibodies that attack type 1 interferons, neutralizing the immune system’s ability to block the SARS-CoV-2 virus, were present in 10% of patients with life-threatening coronavirus diseases 2019 (COVID-19) pneumonia, a new study found.

New data may evidence oral care as a therapeutic measure to reduce bacterial and viral spread.

The hospital found just 2 cases in their first 12 weeks were borne in the facility. A study author provides insight.
An investigator shares detail into how the neutralizing, immune-signaling molecule may provide continued success against the virus.