Respiratory Infections
Latest News
Latest Videos

CME Content
More News
Adam Brufsky, MD, explains the insights years of research into SARS-CoV-1 may hold for SARS-CoV-2.

As the debate on hydroxychloroquine rages on, the FDA and a new advocacy group have sparred about the appropriateness of physicians prescribing the drug for COVID-19.

The trial will assess a monoclonal antibody based investigational treatment that may prevent COVID-19.

The VIP antiviral therapy shows capability in reducing COVID-19 inflammatory biomarkers, as well as respiratory failure risks.
Collodial stability measures may predict whether a potential antibody-based treatment will work early on in the drug development process.

Manufacturers announce their supplies are being sent out for the upcoming season.

New findings show "structural inequities" among the US and UK frontline responders first infected months ago.

Dr. Anthony Fauci, CDC's Dr. Robert Redfield, & HHS' Admiral Brett Giroir spoke at a congressional hearing on coordinating the future of the national coronavirus response.
A look at the history of vaccines, our society's current standing in public health and vaccine preparedness, and what may come in the near future.

What role does new research play in combating public vaccination concerns?

Peter Hotez, MD, PhD, recently authored a paper proposing a federally directed but state-adaptive reopening that aims for acceptable national suppression by October 1.
A discussion on the process by which the FDA regulates vaccine candidates at a time when COVID-19 candidates are progressing.
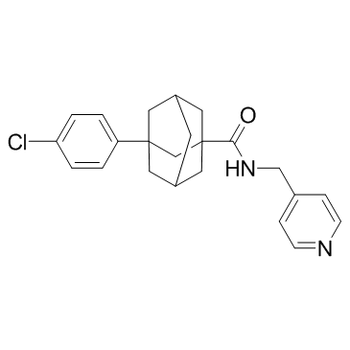
RedHill Biopharma has initiated a global phase 2/3 study in order to evaluate the use of opaganib in patients with severe COVID-19.

On Tuesday, Cain was said to be recovering, possibly reflecting the complex immune system stages of COVID-19 disease course.
Why reducing pandemic research and response to yes-or-no outcomes is harming public health.

People with travel links to China, Iran, and Italy may have accounted for nearly two-thirds of early COVID-19 cases in affected countries.

The method extracts SARS-CoV-2 RNA with the Mag-Bind Viral RNA Xpress Kit and the Hamilton MagEx STAR.
Dr. Adam Brufsky, an oncologist, compares viral and cancer immunity. Dr. Patrick Soon-Shiong connects the relation to COVID-19 vaccine research.

What should be anticipated in effectiveness and implication for the leading candidates.

What happens if we don't get a COVID-19 vaccine? Patrick Soon-Shiong, MD, describes another potential route for research efforts, which could also supplement vaccine development.

A cohort assessment of New York-based deliveries from SARS-CoV-2 positive mothers showed no infants were infected nor symptomatic after 2 weeks.

Dr. Patrick Soon-Shiong and Dr. Adam Brufsky move on from the messenger RNA candidates to the adenovirus vaccines.

Dr. Patrick Soon-Shiong, Chairman and CEO of ImmunityBio, explains why T-cell immunity could be an important metric when evaluating experimental COVID-19 vaccines.

Stroke associated with SARS-CoV-2 infection appears to be more dangerous for patients relative to the baseline risk from stroke, according to a study published earlier this month.

There is mixed international evidence about whether returning to school results in increased transmission or outbreaks, authors explained, and surges may be multiply determined.