
Experts suggest the vaccine, if regulated by the FDA, would benefit the US as a booster dose to combat waning immunity against emerging variants.

Experts suggest the vaccine, if regulated by the FDA, would benefit the US as a booster dose to combat waning immunity against emerging variants.

The newest data for the real-world assessment show the mRNA vaccine provides greater immunogenicity in persons aged 12-15 years old than in young adults.
TAK-003, from Takeda, is showing slightly improved protection among seropositive participants through its 36-month follow-up.
A concerted effort to increase flu vaccination during the COVID-19 pandemic resulted in more protection among a high-risk population, as well as educational opportunities for pharmacy students.
Discriminately affected populations face a wall of hurdles, historic and contextual, in buying into COVID-19 vaccine benefit. What can clinicians and public health officials do to help?

The first US COVID-19 vaccine breaks ground again, as the first authorized for patients as young as 12 years old.

New real-world research suggest the vaccine may help provide control of the pandemic virus.

A treatment arm of up to 3000 participants aged 12-17 years old will be rolled into the North American-based PREVENT-19 trial.

New analysis of registry data suggest pregnant persons are not facing particularly worse pregnancy nor neonatal outcomes after mRNA vaccination.

An interventional cardiologist discusses the observed cases of cerebral venous thrombosis in women administered the Johnson & Johnson COVID-19 vaccine.

Carlos del Rio, MD, discusses the United States' pandemic situation in assessing the blood clot events observed in 6 Johnson & Johnson vaccine recipients.

Jason Gallagher, PharmD, adds context behind the FDA and CDC's decision to pause and review blood clotting events in 6 women given the company's COVID-19 vaccine.

The federal agencies will advise states withhold administration of the Johnson & Johnson one-shot product while investigating risk of a rare blood clot disorder.
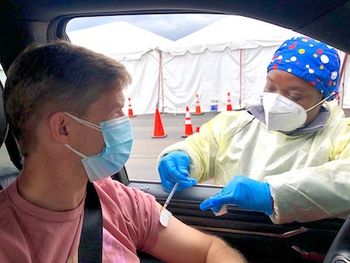
Our editor-in-chief writes on the role caregivers play in informing the public on the perks of a more vaccinated population.

The newest research of rare adverse events among patients administered the vaccine highlights that venous or arterial thrombosis can develop in unexpected regions, including the brain or abdomen, 5-20 days after administration.
A new commentary from experts highlight the evolving understanding of antibody response in vaccinated transplant patients.

An expert stresses the need to consider individuals' worry over information—and even their history with systemic biases.

Vaccine access and new cases are both increasing. An expert discusses earning trust in at-risk communities.
Health regulators in the UK have now found 30 cases involving rare blood clot events after the administration of the AstraZeneca COVID-19 vaccine, but still recommend its use.

The 2 revisions made to the EUA pertain to the number of doses allotted to the current vials containing the vaccine, and allow a new vial that has a maximum of 15 doses.

The companies are now seeking a Biologics License Application with the FDA, which would allow it to become more greatly available for adults in the US.

The pivotal phase 3 data, based off 18 reported cases among placebo patients, includes the companies' intention to request authorization for adolescents aged 12-15 years old.

Cases have increased by 16%, while 50 million have now been fully vaccinated. Federal leadership is asking Americans to hold on longer.

A statement hours after the company reported promising interim phase 3 data stated the Data Safety and Monitoring Board believes they included outdated data “which may have provided an incomplete view of the efficacy data.”