
The VRBPAC committee voted 17-4, with 1 abstention, to support the benefit-risk profile of BNT162b2 for preventing COVID-19 in persons aged 16 years and older.

The VRBPAC committee voted 17-4, with 1 abstention, to support the benefit-risk profile of BNT162b2 for preventing COVID-19 in persons aged 16 years and older.

The first COVID-19 vaccines in the US will be from the unique platform. An expert explains how they work.

The updated vaccine efficacy and safety outcomes include 37,000-plus participants from the ongoing, multi-national trial.

Here is everything you need to know about the second vaccine submitted for an EUA this year.
A look at the logistical questions which remain with the two leading candidates.

Here is what you need to know about BNT162b2, possibly the first vaccine regulated for COVID-19 prevention.
More than 13 million children did not receive their first DTP vaccine dose in 2019—and investigators anticipate the 2020 rate will be worse.
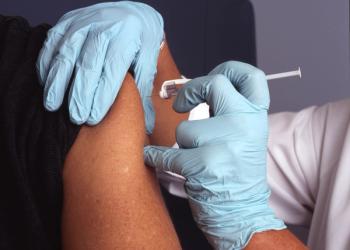
Data show none of the treated volunteers to develop COVID-19 experienced a severe form of the disease, versus 11 given placebo.

The Vanderbilt Professor of Preventive Medicine shares thoughts on the first potential COVID-19 vaccine, at a time of record new daily cases.
The biotechnology has reached it COVID-19 case accrual mark, and announced plans to submit data to an independent review board.

The company is still awaiting its threshold for total COVID-19 infections in its 44,000-patient assessment. Nonetheless, they believe they have made history.
We asked a simple question to our IDWeek experts: has COVID-19 taken attention away from any greater infectious disease issues?

Vaccination for VZV and HPV requires special consideration in immunocompromised patients. They are at risk for more severe viral illnesses if not immune, but they may also have decreased response to, and increased adverse effects from, vaccines.

A glimpse into FDA advisor perspective just weeks before EUAs are anticipated to be sent.
The presenting IDWeek author discusses the implication of promising phase 3 data for a new HBV vaccine.

The conclusion to the independent safety review punctuates a 50-day period of scrutiny and discussion around 2 non-fatal adverse events reported in patients.
In an effort to expand serotype coverage beyond 13, a 20-valent pneumococcal conjugate vaccine, PCV20, is currently in development.
New head-to-head phase 3 data show a promising potential new HBV vaccine may join the market in the near future.

A vaccine developer joins for a firsthand discussion on the development, research, and distribution process.

Pfizer anticipates to have completed FDA-mandated safety data a month from now. An EUA application should shortly follow.

The Meharry and Vanderbilt-based expert shares insights into reaching less trustful populations on the first podcast episode.
In consideration of the idea that the pandemic may lead to greater knowledge and adoption of preventive health measures.

Numerous candidates are in late-stage assessment. An expert shares what the diverse agents should show before being considered for regulation.