
The CDC’s plan for testing patients with flu for coronavirus will inform their response strategy, should the infection spread, the agency said.

The CDC’s plan for testing patients with flu for coronavirus will inform their response strategy, should the infection spread, the agency said.
The Advisory Committee on Immunization Practices has announced changes to the 2020 child and adolescent vaccine schedule.

The NIH has begun the first clinical trial evaluating the safety of a monthly dapivirine ring used to prevent HIV among pregnant women in eastern and southern Africa.

A commentary agrees with World Health Organization recommendations against routine use of corticosteroids in treatment of COVID-19.

A new study has identified that misinformation and limited clinician communication around HPV risk among young men who have sex with men some of the barriers to HPV vaccination.

Here is a look at infectious disease-related US Food and Drug Administration (FDA) news from the week of February 9, 2020.

We’ve rounded up a list of important US Food and Drug Administration (FDA) and US Department of Agriculture (USDA) recalls from this past week.

Stay up-to-date on the latest infectious disease news by checking out our top 5 articles of the week.
Pneumococcal conjugate vaccines have been effective at reducing pneumonia, sepsis, and bacteremia in immunized populations. Pneumococcal meningitis, however, remains a significant challenge.

People living with HIV who don’t receive early intervention and primary care end up with a disproportionate share of health care costs, according to a new study that examined barriers to care and costs at a Dublin hospital.
Social and biological factors increase the risk of respiratory failure and death in vulnerable pre-term infants, a new study shows.
PWID are unlikely to receive testing for HIV and hepatitis C, according to a new study that found that 8.5% were tested for HIV and 7.7% were tested for HCV within 1 year of a clinical encounter consistent with injection drug use.
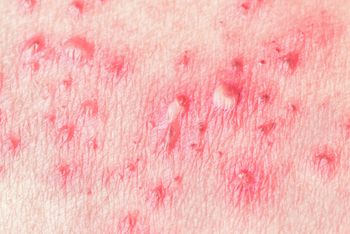
A new study found that the Zoster Vaccine Live not only reduced the risk for shingles but also reduced stroke risk by 16% in older adults.

The rising price of antiretroviral therapy for people living with HIV in the United States is a barrier to adherence. It also blunts efforts to achieve higher rates of viral suppression.

The International Health Regulations Emergency Committee decided today that the Ebola outbreak in the Democratic Republic of the Congo remains a PHEIC.

In a scenario where health care preparedness is key, what are the best strategies for US hospitals to pursue?
There could be viable options within a few months, but will it be in time to contain an outbreak?

A new article examines pregnancy outcomes among women who received the rVSV-ZEBOV vaccine in the STRIVE study.

With cases of the novel coronavirus (COVID-19) increasing across the globe, why should we be paying attention to influenza—a respiratory illness that is seen year after year?
Less than 1 in 4 teenage boys considered at-risk ever received an HIV test in a recent study.
A new retrospective study provides more evidence that a single HPV vaccine dose may be effective in preventing cervical cancer.

The fact remains that this strain of coronavirus is not highly contagious; it behaves very similarly to other infectious viruses by targeting mainly the weak and/or immunocompromised portions of the population.
The PRIMVAC placental malaria vaccine candidate was found to be safe and immunogenic.
A phase 1 clinical trial suggests that the m102.4 antibody is well tolerated and safe for use as a treatment against henipaviruses.

Does employing the universal use of gloves and gowns decrease the acquisition of antibiotic-resistant gram-negative bacteria?

Here is a look at infectious disease-related US Food and Drug Administration (FDA) news from the week of February 2, 2020.

We’ve compiled a list of recalls issued by the US Food and Drug Administration (FDA) and US Department of Agriculture (USDA) from this past week:

Screening for 2019 novel coronavirus continues around the world. A new research letter on the clinical characteristics of 13 patients sheds light on the nature of cases outside of Wuhan, China.

Stay up-to-date on the latest infectious disease news by checking out our top 5 articles of the week.
Despite new treatment options with a lower theoretical risk, approximately 40% of patients with HCV experienced a drug-drug interaction.