Bacterial co-infections with COVID-19 are uncommon, but frequent use of antibiotics has fostered treatment resistance in co-occurring and secondary infections.

Bacterial co-infections with COVID-19 are uncommon, but frequent use of antibiotics has fostered treatment resistance in co-occurring and secondary infections.

The 2023 changes include vaccines for influenza, pneumococcal disease, measles, mumps, and rubella (MMR) and COVID-19.

Spherical hydrogel inhalation for enhanced lung defense (SHIELD) is an investigational inhalable that coats the airways and serves as a physical barrier against COVID-19.

This week's most-read story described healthy lifestyle choices that are proven to decrease the risk of "long COVID."

The antiviral did not modify SARS–CoV-2 RNA clearance, and did not increase the risk of adverse events.
The vaccine even provided significant protection when children had just one dose, investigators found.
After an 18-month study, investigators determined none of the diseases examined were more frequent among the vaccinated than among the non-vaccinated.
A phase 1 trial with the investigational vaccine, TNX-801, for the prevention of mpox and smallpox is expected to start in the second half of 2023.
New research identified the epidermal growth factor receptor (EGFR) is essential for hepatitis E to enter cells.
A single-dose of peginterferon lambda demonstrated efficacy in a largely vaccinated cohort and the therapy accelerated clearance of the virus.

Chinese investigators said genome analysis suggests 2 existing Omicron sub-variants, BA52 and BF7, were among the most dominant variants in Beijing.
International research efforts developed recommendations to optimize, control, and validate quantitative viral DNA measurements of chronic hepatitis B in the liver.
Implementing rapid near patient testing (NPT) reduced patient isolation time, length of hospital stay, antibiotic usage, and overall cost.

Infectious disease outbreaks will continue to increase and trying to find strategies to ensure providers are cared for to prevent burnout and shortage staffing is essential if another pandemic arises.

Among male couples, HIV PrEP usage is suboptimal. Lingering stigma may be preventing men who have sex with men from using PrEP, even when in a relationship with an HIV-positive partner.

A prominent researcher offers some insights on this emerging fungal infection.

February 7 is National Black HIV/AIDS Awareness Day (#NBHAAD). In observance, we're recapping stories to raise awareness and work toward ending HIV/AIDS disparities in Black communities.

Antibiotics are apparently not the only widely used pharmaceuticals that can induce emergence of treatment resistant bacteria.
Women who made healthy lifestyle choices prior to contracting COVID-19 had a 49% reduced risk of long COVID.

FDA says products have the potential to be contaminated with Listeria and were distributed along the east coast in several states.
New research suggests the majority of people hospitalized with influenza also had a chronic illness.
Point-of-care RNA viral load testing is associated with shorter times between testing and treatment initiation and higher treatment uptake, a recent study found.
Looking to appeal to the financial incentives of antibiotic development, a recent policy paper explores how lives can be saved and a return of investment (ROI) can be realized.
Treating Clostridioides difficile infection is complicated in patients coinfected with COVID-19. However, a new study suggests a significant benefit of fecal microbiota transplant (FMT) in these coinfected patients.

In doing so, the country met the new target of the World Health Organization.

The biggest COVID-19 news from the past week.

Can a similar approach that brought breakthrough therapies for cystic fibrosis bring about much needed change to antibiotic development?
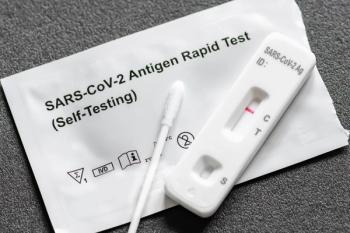
The vast majority of COVID-19 infections are diagnosed at home, with rapid antigen tests. How has this affected public reports of COVID-19 prevalence?
The microbiome may be a source of future investigation and modulation approaches, the study authors wrote.
New York City has ended their mpox outbreak after months of successful community outreach and vaccination campaigns.