
An interventional cardiologist discusses the observed cases of cerebral venous thrombosis in women administered the Johnson & Johnson COVID-19 vaccine.

An interventional cardiologist discusses the observed cases of cerebral venous thrombosis in women administered the Johnson & Johnson COVID-19 vaccine.

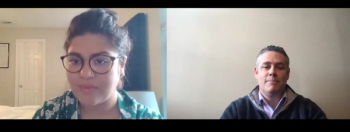
Carlos del Rio, MD, discusses the United States' pandemic situation in assessing the blood clot events observed in 6 Johnson & Johnson vaccine recipients.

Jason Gallagher, PharmD, adds context behind the FDA and CDC's decision to pause and review blood clotting events in 6 women given the company's COVID-19 vaccine.
A recent study looked at the racial disparities amongst people living with HIV and COVID-19 positivity rates.

An expert stresses the need to consider individuals' worry over information—and even their history with systemic biases.

Vaccine access and new cases are both increasing. An expert discusses earning trust in at-risk communities.
Long-acting injectables for latent and active tuberculous (TB) might be a treatment option in the future and could open up the door to possibly bundling HIV and TB care together.
To mark World TB Day, a pair of experts break down the currently defined regimens for latent and active tuberculosis—and what may still come.
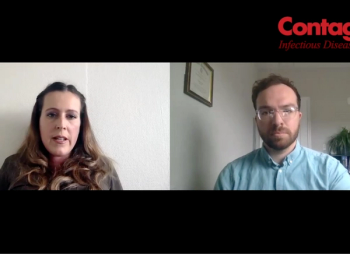
The small country's emphasis on accessible testing provided more up-to-date tracking, and opportunity for research into reinfection risk.
An interview on perspectives learned from a nationwide PCR test database assessment.
A bamlanivimab study showed a 72% reduction in the incidence of COVID-19 and an 80% reduction of progression in the virus in residents in assisted living facilities and nursing staff.

While at the earliest stages of potential development, long-acting/extended release (LA/ER) formulations for treatment and prevention of TB could be something to be studied in the future.

During the pandemic, reduced HIV testing led to concerns about getting people diagnosed and on antiretroviral therapy (ART), and achieving viral suppression (VS).
ViiV Healthcare’s investigational therapy, GSK’254, demonstrated antiviral activity, safety, and tolerability in its phase 2a proof-of-concept findings.

With the highest HIV incidence rate in this part of the country, the need to get people from diagnosis to timely antiretroviral therapy (ART) remains an urgent need.
Merck has developed its investigational islatravir subdermal implant for prevention of HIV infection, and it is going to be studied for a long-term delivery of up to 1 year.
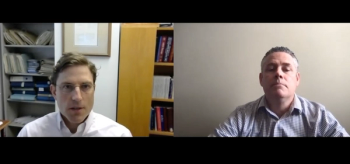
How observed changes in SARS-CoV-2 lineage have warranted heightened priority, and why sequencing deserves better resourcing beyond the pandemic.
A team of cross-country investigators discuss their coordinated tracking of a new variant in the US earlier this year.

A talk on optimistic, but pragmatic hopes for HIV cure and vaccine research, led by federal-level experts.

Attention to the endemic virus has lessened over years, and screening and prevention access remains limited among the most at-risk groups.

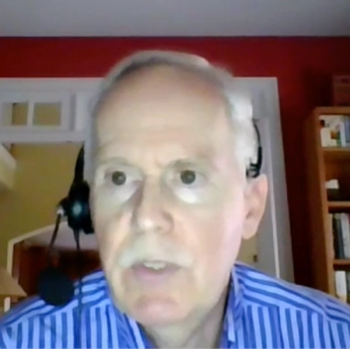
A John Hopkins expert and major study leader details the evolving epidemiology of the virus.

CalciMedica has developed Auxora, a calcium release-activated calcium (CRAC) channel inhibitor, and it has shown it can reduce the levels of D-dimer, a key biomarker associated with COVID-19 mortality.
A video breakdown of the past week's developments and news in leading vaccine candidates.
Current trial evidence is severely lacking for the at-risk group, but does clinical history suggest safety?
An interview on new survey data suggesting a majority of fertility and pregnant patients are not interested in the currently available vaccines.
The ability to monitor, mitigate, and prevent new variant spread could influence the odds of majority immunity.
More transmissible variants are emerging quickly. An expert explains why it's expected, but concerning.
Athersys has developed its MultiStem cell therapy for COVID-19 Acute respiratory distress syndrome (ARDS). Athersys CEO Gil Van Bokkelen, PhD, and CFO Ivor Macleod, recently spoke to Contagion providing background on the company and their MultiStem therapy.
In consideration of the idea that the pandemic may lead to greater knowledge and adoption of preventive health measures.
Perspective on the ongoing independent safety reviews for one of the more promising vaccine candidates.