Last influenza season was notably quiet during peaking COVID-19 cases. Experts explain what may influence risk of dueling epidemics this year.
Kevin Kunzmann is the managing editor for Contagion, as well as its sister publication HCPLive. Prior to joining parent company MJH Life Sciences in 2017, he worked as a health care and government reporter for The Pocono Record, and as a freelance writer for NJ Advance Media, The Express-Times, The Daily Journal, and more. He graduated from Rowan University with a degree in journalism in 2015. In his spare time, he enjoys reading, cooking, running his dog, and complaining about the Mets. Follow him on Twitter @NotADoctorKevin or email him at kkunzmann@mjhlifesciences.com

Last influenza season was notably quiet during peaking COVID-19 cases. Experts explain what may influence risk of dueling epidemics this year.

Pfizer and Moderna will include children aged 5-11 years old in ongoing trials to speak toward risk of rare adverse events observed in other young recipients.

A discussion as to why people with unstable housing living with HIV may not prefer telehealth versus consistent in-person care.
The risk of COVID-19 severity in people living with HIV may be less than previously anticipated.
Phase 3 trials for islatravir are underway. What are investigators considering with the Merck investigative drug?
New 24-week findings from Merck highlight the potential of a 1-month PrEP option for low-risk patients.
The pandemic's effect on in-person care prioritization may have indirectly influenced the rate of screening opportunities in at-risk persons.
New findings from CUSTOMIZE suggest the once-monthly injection therapy was feasibly implemented and generally favored to once-daily tablets.
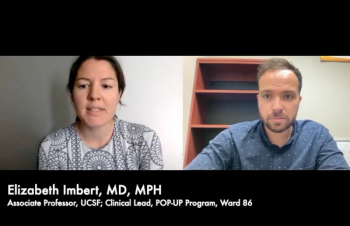
Perspective gained from persons treated a real-world clinic may help better inform strategies to treat an HIV population that often struggles with viral suppression and consistent care.
A new study from IAS 2021 suggests persons with HIV hospitalized for COVID-19 do not face worse mortality nor major cardiovascular event risk.

The prophylaxis showed non-inferiority across shared serotypes with an available 13-valent vaccine in its indication-supporting data.

A meeting between developers and federal experts was inconclusive to the necessity of a third mRNA dose for COVID-19.

The announcement aligns with an FDA and CDC statement against the need for booster doses.

Seqirus is seeking reactogenicity and immunogenicity outcomes between its influenza vaccines and available mRNA vaccines for COVID-19.

Plus: how could the COVID-19 testing market boom improve public flu awareness?

COVID-19 response restrained flu outbreaks last year. An expert explains his concern that won't be enough this winter.

An interview with a study author on new Novavax-Seqirus co-administration trial data, and the future concerns of circulating COIVD-19 and influenza.
New ASM data show indoor environments are associated with quick and frequent pathogen contamination of the common respiratory device.

An interview with a study author on unique associations between county infrastructures and COVID-19 fatality rates.

The device may help clinicians differentiate between past infections and co-infections during the upcoming flu season.

New findings on an available 15-minute test show an approximate 81% sensitivity in detection of SARS-CoV-2.

New findings from the ASM 2021 World Microbe Forum suggest the emergency-authorized platform can capably detected the highly transmissible UK-borne variant.

Experts suggest the vaccine, if regulated by the FDA, would benefit the US as a booster dose to combat waning immunity against emerging variants.

The authorization change halves the required dose of the monoclonal antibody regimen, based on recent data.

A prospective study suggest co-infections and secondary infections are infrequent among COVID-19 patients, warranting the need for improved stewardship.
New research from Emory show a significant difference in institutional antibiotic prescribing after education on the value of PCT testing in infected patients last year.
An assessment of the frontline agents show similarities infection cure and recurrence rates at 30 days.

The newest data for the real-world assessment show the mRNA vaccine provides greater immunogenicity in persons aged 12-15 years old than in young adults.
TAK-003, from Takeda, is showing slightly improved protection among seropositive participants through its 36-month follow-up.
An effort to increase stewardship showed that 5-day and 10-day prescription stop dates for antibiotics did little to influence actual therapy length and outcomes.