
An assessment from the Cleveland Clinic shows prescribing concordant to guidelines can exceed 60% in certain facilities.
Kevin Kunzmann is the managing editor for Contagion, as well as its sister publication HCPLive. Prior to joining parent company MJH Life Sciences in 2017, he worked as a health care and government reporter for The Pocono Record, and as a freelance writer for NJ Advance Media, The Express-Times, The Daily Journal, and more. He graduated from Rowan University with a degree in journalism in 2015. In his spare time, he enjoys reading, cooking, running his dog, and complaining about the Mets. Follow him on Twitter @NotADoctorKevin or email him at kkunzmann@mjhlifesciences.com

An assessment from the Cleveland Clinic shows prescribing concordant to guidelines can exceed 60% in certain facilities.
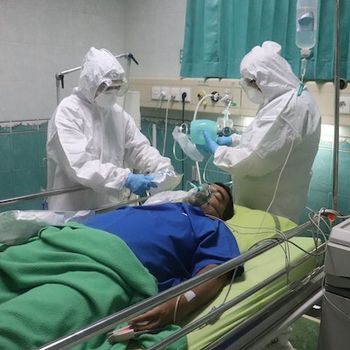
The IL-6 targeting monoclonal antibody is linked to improved inflammatory and hemodynamic markers in hospitalized patients.
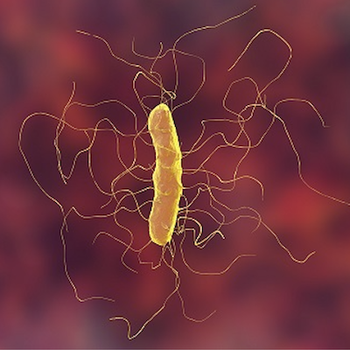
A meta-analysis suggest the 2 first-line C diff therapies provide similar outcomes, yet differ in recurrent infection risk.
A concerted effort to increase flu vaccination during the COVID-19 pandemic resulted in more protection among a high-risk population, as well as educational opportunities for pharmacy students.

With yesterday’s announcement, a lot of questions remain about how to proceed. One expert weighs in on this significant topic including how masks have substantially helped as a COVID-19 prevention strategy.
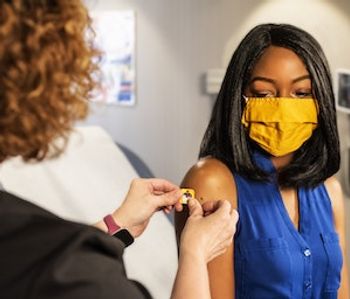
Discriminately affected populations face a wall of hurdles, historic and contextual, in buying into COVID-19 vaccine benefit. What can clinicians and public health officials do to help?

The first US COVID-19 vaccine breaks ground again, as the first authorized for patients as young as 12 years old.

New real-world research suggest the vaccine may help provide control of the pandemic virus.

A treatment arm of up to 3000 participants aged 12-17 years old will be rolled into the North American-based PREVENT-19 trial.

New analysis of registry data suggest pregnant persons are not facing particularly worse pregnancy nor neonatal outcomes after mRNA vaccination.

A cardiology expert discusses the unique and currently investigated relationship between COVID-19, adenovirus vaccines, and thrombotic events.

The decision follows emerging data showing prevalent virus variants are unaffected by the monoclonal antibody.

New data indicates failed parasite clearance among Rwandan children is linked to a mutation also observed in southeast Asia.

An interventional cardiologist discusses the observed cases of cerebral venous thrombosis in women administered the Johnson & Johnson COVID-19 vaccine.

Carlos del Rio, MD, discusses the United States' pandemic situation in assessing the blood clot events observed in 6 Johnson & Johnson vaccine recipients.

Jason Gallagher, PharmD, adds context behind the FDA and CDC's decision to pause and review blood clotting events in 6 women given the company's COVID-19 vaccine.

The federal agencies will advise states withhold administration of the Johnson & Johnson one-shot product while investigating risk of a rare blood clot disorder.
New cohort analyses show the popular UK variant is linked to a higher viral load, though patients have not experienced worse likelihood of outcomes.

The newest research of rare adverse events among patients administered the vaccine highlights that venous or arterial thrombosis can develop in unexpected regions, including the brain or abdomen, 5-20 days after administration.
A new commentary from experts highlight the evolving understanding of antibody response in vaccinated transplant patients.
An outbreak which began at an infected healthcare worker's funeral has resulted in nearly 100 contact follow-ups.

An expert stresses the need to consider individuals' worry over information—and even their history with systemic biases.

Vaccine access and new cases are both increasing. An expert discusses earning trust in at-risk communities.

The companies are now seeking a Biologics License Application with the FDA, which would allow it to become more greatly available for adults in the US.

The pivotal phase 3 data, based off 18 reported cases among placebo patients, includes the companies' intention to request authorization for adolescents aged 12-15 years old.

Cases have increased by 16%, while 50 million have now been fully vaccinated. Federal leadership is asking Americans to hold on longer.

A new cohort assessment evidences a disparity in weight-related outcomes with the ART regimens at 18 months.

A month-long assessment in Wuhan sought the human origin of SARS-CoV-2. The investigators graded 4 possible scenarios.
To mark World TB Day, a pair of experts break down the currently defined regimens for latent and active tuberculosis—and what may still come.

A statement hours after the company reported promising interim phase 3 data stated the Data Safety and Monitoring Board believes they included outdated data “which may have provided an incomplete view of the efficacy data.”