
Though regulatory authorization has not been granted, the company intends to vaccinate up to 50 million people prior to the Lunar New Year.
Kevin Kunzmann is the managing editor for Contagion, as well as its sister publication HCPLive. Prior to joining parent company MJH Life Sciences in 2017, he worked as a health care and government reporter for The Pocono Record, and as a freelance writer for NJ Advance Media, The Express-Times, The Daily Journal, and more. He graduated from Rowan University with a degree in journalism in 2015. In his spare time, he enjoys reading, cooking, running his dog, and complaining about the Mets. Follow him on Twitter @NotADoctorKevin or email him at kkunzmann@mjhlifesciences.com

Though regulatory authorization has not been granted, the company intends to vaccinate up to 50 million people prior to the Lunar New Year.

The agreement could mean upwards of 70 million more doses for eligible patients in mid-2021.
Though research is pending, the CEO used simple science to explain why the mRNA vaccine may still prevent from the UK variant.
A new case series report highlights the unique circumstances of infant illness associated with COVID-19.
New data suggests informed, practical pooled testing could improve both public health response and clinical assessment of the pandemic.

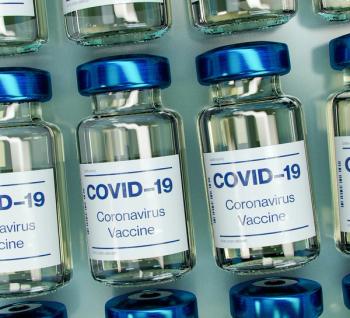
Up to 40% more doses—or, enough to vaccinate another person with two doses—has been observed in the vials distributed at the beginning of this week.

The country's first authorized vaccine will be first available to healthcare workers and long-term care residents.

The VRBPAC committee voted 17-4, with 1 abstention, to support the benefit-risk profile of BNT162b2 for preventing COVID-19 in persons aged 16 years and older.

The first COVID-19 vaccines in the US will be from the unique platform. An expert explains how they work.

The updated vaccine efficacy and safety outcomes include 37,000-plus participants from the ongoing, multi-national trial.

Here is everything you need to know about the second vaccine submitted for an EUA this year.
A look at the logistical questions which remain with the two leading candidates.
Articles not undergoing peer review are reaching widespread dissemination during the pandemic. What are the implications?
A study author at Keck Medicine of USC discusses an ongoing assessment of LY-CoV555 for preventing severe COVID-19, and the overall benefit of the drug class for pandemic mitigation.
The IDSA representative discusses advances made in recent years, and how foundational guidance could be set by the FDA with input from specialists.
The former FDA commissioner weighs in on the best measures to mitigate skyrocketed new cases, and his timeline for national recovery.

The influenza therapy is expanded to include people who have been exposed to the flu.

The third EUA granted to an investigational COVID-19 therapy regimen is indicated for preventing progression from mild, to severe symptoms.

Here is what you need to know about BNT162b2, possibly the first vaccine regulated for COVID-19 prevention.

For the first time, prescribed users at suspected risk of COVID-19 can receive rapid results from home.
More than 13 million children did not receive their first DTP vaccine dose in 2019—and investigators anticipate the 2020 rate will be worse.
Phase 2 data hints the immune response-modulating therapy could play a role in burdened hospital settings.
Data show none of the treated volunteers to develop COVID-19 experienced a severe form of the disease, versus 11 given placebo.

The Vanderbilt Professor of Preventive Medicine shares thoughts on the first potential COVID-19 vaccine, at a time of record new daily cases.
The biotechnology has reached it COVID-19 case accrual mark, and announced plans to submit data to an independent review board.

Findings from Michigan show financial, mental health, and cardiopulmonary impacts are prevalent in patients 2 months after hospital care.

The new research letter also highlights a concerning rate of infection among Latino patients.

The company is still awaiting its threshold for total COVID-19 infections in its 44,000-patient assessment. Nonetheless, they believe they have made history.

A new survey shows just 2% of children with rheumatic disease reported COVID-19 positive test results or symptoms through early May.