
Following implementation of stewardship program-guided blood culture communication process, the time to optimal therapy was 9.2 hours shorter for patients at Saint Luke’s Hospital.

Following implementation of stewardship program-guided blood culture communication process, the time to optimal therapy was 9.2 hours shorter for patients at Saint Luke’s Hospital.
Antibiotic prescription following ventilation in children with RSV-LRTI was associated with a 1.21-day shorter duration of ventilation and a 2.07-day shorter length of hospital stay.

The FDA has accepted New Drug Applications for oral and IV formulations of Nabriva Therapeutics’ lefamulin for the treatment of community-acquired bacterial pneumonia.

The FDA has approved room temperature stable, premixed vancomycin injections, which will be available in ready-to-use bags.

The FDA has approved triclabendazole (Egaten) for the treatment of fascioliasis, a neglected tropical disease in patients 6 years of age and older

The FDA has authorized a phase 2 study of PUR1900, an inhaled formulation of itraconazole, for the treatment of allergic bronchopulmonary aspergillosis.

The NIH has announced that a trial evaluating the safety of and adherence to a vaginal ring containing dapivirine and oral tablets for PrEP is now underway in southern Africa.

Health officials announced that individuals who consumed raw milk from Miller’s Biodiversity Farm may have been exposed to a drug-resistant strain of brucellosis.
Pharmacists from across the US have authored a special feature to provide advice to clinicians looking to implement penicillin allergy skin testing (PAST) at their institution.

The US Food and Drug Administration has granted Breakthrough Therapy Designation to investigational drug CP101 for the treatment of patients with recurrent C diff infection.
Although the majority of states grant vaccine exemptions for medical reasons and religious beliefs, 17 states allow exemption for personal or moral beliefs, increasing the likelihood of outbreaks of preventable diseases like measles.
Paratek Pharmaceuticals has announced the launch of omadacycline, including 3 antimicrobial susceptibility tests and a surveillance program.

A phase 3 trial has been approved for SPR994, a candidate being developed as an oral carbapenem antibiotic for the treatment of cUTIs.

The FDA has accepted an NDA for imipenem/cilastatin/relebactam as well as a supplemental NDA for ceftolozane/tazobactam.

Rutgers University investigators created a pictorial key that anyone with a strong enough microscope can use to correctly identify the Asian longhorned tick from other harmless species.

SCYNEXIS has announced results from the first interim analysis of the phase 3 FURI study evaluating oral ibrexafungerp for invasive fungal infections.
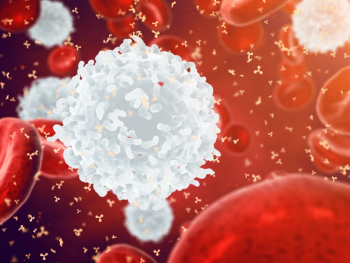
Basophils play a critical role in inducing immune responses against infections and could potentially play a part in preventing the development of sepsis.
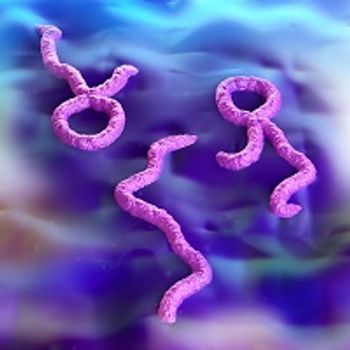
Investigators have detected the presence of Ebola in a bat in Liberia, revealing more about how bats serve as reservoirs for the deadly pathogen.

Merck has received a Breakthrough Therapy Designation for investigational 15-valent pneumococcal conjugate vaccine V114.

Thomas Lodise, PharmD, PhD, explains the economic burden of community-acquired pneumonia as well as the associated risks that accompany currently available treatment options.
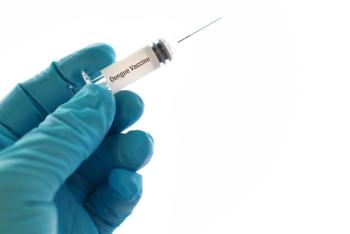
TAK-003, being developed by Takeda, is a tetravalent vaccine based on a live-attenuated dengue serotype 2 virus, which provides the genetic “backbone” for all 4 virus serotypes.

Eleven cases of Salmonella Typhimurium have been reported in a multistate outbreak with a suspected link to contact with hedgehogs.

CDC reports that 81 individuals brought measles into the United States following a trip to another country in 2018.
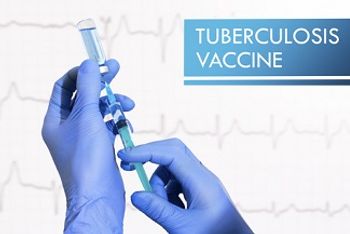
A clinical trial is underway evaluating whether the vaccine candidate ID93 + GLA-SE can induce immune responses to prevent against reinfection and reactivation of TB.

WHO has compiled a list of the top 10 threats to global health in 2019 and 6 of them are infectious disease-related.

The FDA has approved the use of the 0.5 mL dose of Fluzone Quadrivalent influenza vaccine to include children age 6 through 35 months.
As the government shutdown passes the 1-month mark, Contagion® investigates how it is affecting public health agencies.

Authors of a new report in JAMA introduce the 4 moments of antibiotic decision making, an easy-to-remember method that can be incorporated in real-time clinical practice.

In a multi-institution collaboration assessing 22 influenza forecasting models, the majority of models consistently showed higher accuracy than historical baseline models.

We’ve rounded up a list of important US Food and Drug Administration (FDA) and US Department of Agriculture (USDA) recalls from this past week.