
Here’s what needs to be addressed in order to make real progress in the fight against antimicrobial resistance, according to Dr. Ramanan Laxminarayan, PhD, Center for Disease Dynamics, Economics, & Policy.

Here’s what needs to be addressed in order to make real progress in the fight against antimicrobial resistance, according to Dr. Ramanan Laxminarayan, PhD, Center for Disease Dynamics, Economics, & Policy.
The results of a new analysis presented at the 47th Critical Care Congress reveal that incidence of intensive care unit (ICU) bacteremia is decreasing over time.
Only about 1 in 7 HIV-positive individuals are aware of their status.

In case you missed them, we’ve compiled a list of the latest recalls posted this week.

In case you missed them, we've compiled the top 5 articles from this past week.
The results of a new study reveal that hematopoietic cell transplantation (HCT) may still be successful in cases with incomplete radiographic control of invasive fungal infection (IFI) prior to transplant.

While a new study has found that the HPV vaccination rate has more than tripled among young men in the United States, rates for both males and females are still well below public health targets.
Many health care facilities are looking to expand the use of broad-spectrum sporicidal disinfectants beyond patient isolation rooms to better address the role of the environment in pathogen transmission and acquisition.
Antibiotic resistance is highly concerning to researchers, but a team of researchers have identified new synthetic antibiotics with the potential to kill aggressive bacteria.

A new study from Johns Hopkins School of Medicine highlights major racial & ethnic disparities in the diagnosis and treatment of trichomoniasis in the United States.

Public health officials cite zoonotic disease as the top pandemic threat, yet the task of predicting or preparing for a pandemic remains difficult.

SHEA and APIC have declared infection control programs as critical components of antimicrobial stewardship programs—are you including them at your institution?
With influenza B making a late-season rise, health officials are warning that B viruses may cause a second wave of flu this season, while the FDA is backing some alternatives to egg-based flu vaccines.

Based on current projections, antibiotic consumption could increase by as much as 200% by 2030.

This comes on the heels of 2 recent FDA approvals of 2 Mylan antiretrovirals: Cimduo and Symfi Lo.
Antibiotics are the mainstay treatment for CAP; however, the additive role of corticosteroids is continually being debated.
Culture-independent diagnostic tests offer better food-borne infection incidence estimates, but without isolates, laboratories are unable to subtype pathogens, determine antimicrobial susceptibility, and detect outbreaks.

The first documentation of the east Asian tick Haemaphysalis longicornis in the United States has been described in a recent study.

A recent study explores the use of isavuconazole for prophylaxis against invasive fungal infections in allogeneic stem cell transplant patients.
As the leading cause of unplanned 30-day hospital readmissions, sepsis affects more than 1.5 million individuals each year.
A recent study finds that daily antituberculosis therapy is more effective than a thrice-weekly regimen among HIV-positive patients with pulmonary tuberculosis.

Preliminary research suggests that women who take antibiotics for long periods of time in late adulthood may be at increased risk of death from heart disease and other causes.
The vaccine reduced the risk of herpes zoster by 68.2% in high-risk recipients of hematopoietic stem cell transplant.
A new study finds that a 1-month course of antibiotics is as safe & effective as the commonly recommended 9-month course in preventing tuberculosis in those with HIV.

In case you missed them, we’ve compiled a list of the latest recalls posted this week.

Stay up-to-date on the latest infectious disease news by checking out our top 5 articles of the week.
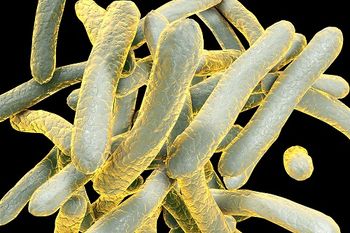
In light of World TB Day, the CDC has released provisional TB surveillance data indicating a decline in cases in the United States, but at a rate that’s too slow to achieve elimination in this century.
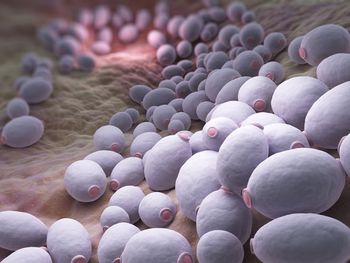
Positive safety and efficacy results have been seen across 2 different dosing regimens as well as for any effects on QT prolongation.

Research presented at the 47th Critical Care Congress reveals that PCT-guided antibiotic cessation in critically ill patients resulted in reduced mortality.
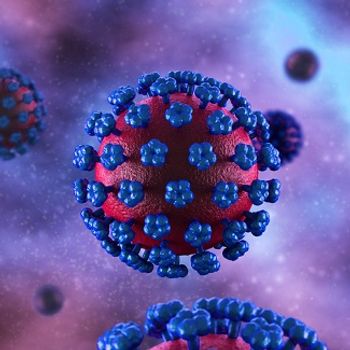
As an outbreak of Lassa fever in Nigeria continues to spread, the Coalition for Epidemic Preparedness Innovations is working with Themis Bioscience to develop a vaccine to protect against the virus.