
Pre-existing conditions were found to increase the risk of COVID-19 related death in those with diabetes.

Pre-existing conditions were found to increase the risk of COVID-19 related death in those with diabetes.
The confusion and uncertainty surrounding the need for a second dose among the public shows the need for better guidance from the medical community.

Patients who received the inhaled glucocorticoid budesonide in the first several days of COVID-19 symptoms were much less likely to require hospital care.

The new report highlights the broad disparities in HIV health among different populations, with Black transgender women facing the highest rates of HIV infection.

This is our inaugural segment for our new video series, 1 Big Question, where we ask the medical community a question about a significant infectious disease topic and get feedback from them.
The most common symptoms were headache, fatigue and soreness at the injection site.
The proportion of cases with CDI in perforated appendicitis was found to be greater in comparison to the non-perforated cases.

A pair of recent studies suggest that the ability of people with blood cancers lymphocytic leukemia and multiple myeloma to produce antibodies in response to COVID-19 vaccines is impaired.

Surveys suggest most students plan to get the shots anyway.

The discovery of these nanobodies may help to create treatments that can protect against COVID-19, as well as future variants of the virus.

Individuals who are fully vaccinated can shed their masks when exercising outdoors and gathering in small groups.

Exposure to a segment of the viral spike protein induced COVID-19 like symptoms and caused lung damage similar to those with the disease.

Even in mild cases, symptoms of COVID-19 can linger for at least 8 months, according to new research.

CDC report highlights disparities among “racial, ethnic, gender, and sexual” minorities.

Treatment with rifampin shows it can increase the chances for recurrent Clostridium difficile (C diff) infection according to investigators in Mexico.

The Biden Administration plans to donate the AstraZeneca vaccines globally upon clearance of safety reviews.
Thailand steps up travel restrictions among a rapidly spreading third wave of the disease.
There have been sporadic cases Hemophagocytic Lymphohistiocytosis, associated with Cytomegalovirus Infection infection, suggesting that CMV may cause clinically significant disease beyond mononucleosis in immunocompetent individuals.

The discovery of C diff’s ability to harness heme to shield itself from the body’s immune responses hopefully brings a new dimension to the fight against the damaging pathogen.
Nursing home residents who had recovered from COVID-19 showed robust antibody levels against the SARS-CoV-2 spike protein after 1 dose of messenger RNA (mRNA) vaccine, a study in France showed.
CDI prevalence among those hospitalized with a COVID-19 infection was not found to be increased despite more use of antibiotics.

The Advisory Committee on Immunization Practices voted 10-4 with 1 abstention to resume the rollout and administration of the Janssen shot.

The 6th major component of the 1-year old ACTIV public-private initiative of the NIH will test candidate drugs to repurpose for COVID-19.

Providing support to communities of color could encourage participation and improve uptake.

Short-course antibiotics are preferable for certain common bacterial infections, including acute bronchitis, pneumonia, urinary tract infection and cellulitis, the American College of Physicians said in a report issuing practice advice.
Experts discuss the bacteria’s transmission in the community setting, how to reduce disease and economic burdens, and ways to improve quality of life for patients.

While the addition of outside air into a building showed a clear benefit, too much air exchange can have a negative impact.

New analysis of registry data suggest pregnant persons are not facing particularly worse pregnancy nor neonatal outcomes after mRNA vaccination.
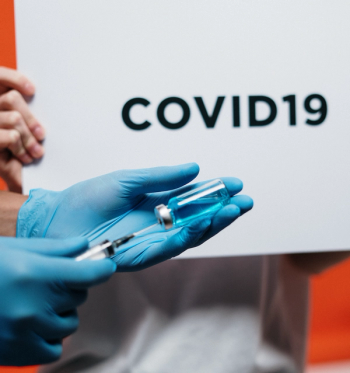
Two cases in the United States showed reinfection after administration of the m-RNA vaccines may complicate protection against new variants, but lend support to a potential need for a booster.

The Johnson & Johnson shot showed an efficacy of 66.9% and 66.1% on the 2 primary endpoints, with a higher efficacy against severe-critical disease.