Although routinely prescribed after surgery, a new meta-analysis of studies on antibiotics and surgery finds there is no need for post-surgery antibiotics if best practices are followed.

Although routinely prescribed after surgery, a new meta-analysis of studies on antibiotics and surgery finds there is no need for post-surgery antibiotics if best practices are followed.

In a recent US survey, African American male participants knew more people infected with COVID-19, had greater knowledge disparities, and were exposed to the virus more than other groups.
Health officials are urging parents to adhere to recommended vaccination schedules amid the COVID-19 pandemic, which has led to a drop in vaccination coverage.

A video review of the latest ID news.

Pregnant women with COVID-19 typically experienced symptoms in their second and third trimester, a new study shows.
Vibeke Strand, MD, MACR, FACP, of the Division of Immunology/Rheumatology at Stanford University School of Medicine, discusses ongoing AI research in her field.
the company announced it COVID-19 medication is looking to address viral replication and uncontrolled inflammatory response.

In a new analysis of Guangzhou city contact tracing data, investigators examined secondary attack rates of SARS-CoV-2.

An overview of 3 recent infectious disease news stories.

A modeling study from the UK reinforces how the transmission rates from infected people can be lowered using a variety of well-known strategies.

Providing oral antibiotics to people who inject drugs with infections may reduce hospital readmissions.

Vibeke Strand, MD, MACR, FACP, discusses patients who are having a hard time getting their usual supplies of HCQ.

A new phase 2b trial shows promise in HCV treatment.

Given there is not a clear international standard for health records, one of the challenges in the early COVID-19 response mobilization was a delayed start to data collection and indexing.
Some countries have been proactive in patient logging and tracking. How can even retroactive assessment benefit future preventive responses?

The technology deploys AI-based algorithms that use machine-learning to identify respiratory failure and hemodynamic instability

New study shows reduced deaths by one-third in ventilated patients, and one-fifth in patients receiving oxygen
Regional disparity in prior authorization requirement for insurance coverage of Pre-exposure prophylaxis (PrEP) for HIV could be discriminatory.

In a modeling study, an estimated 1.7 billion people are at an increased risk of the virus, but a much smaller percentage would require hospitalization.

A look at 3 major headlines in COVID-19 news on June 15, 2020.
In a controlled environment, investigators found sunlight affected the decay rate of the virus.

The anti-malaria drugs—and clinical trials assessing their benefit—have been under close scrutiny since the EUA was first granted in late March.

During the spring COVID-19 outbreak, an examination of Swiss people in Geneva shows only a small amount of the population with IgG antibodies.

Findings show states who followed stay-at-home orders indeed flattened their curves. Now what?
Household members of patients with drug-resistant tuberculosis often have resistant infections, but differences with respect to specific drugs are common.
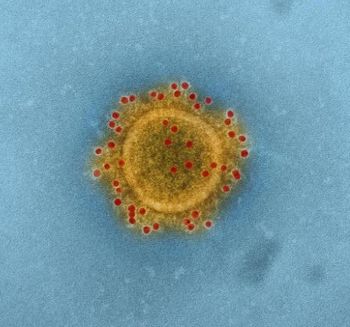
Analysis projects significant toll from virus, even with some measures in place.

The US FDA is a key global regulatory authority. Here are updates from this week on the FDA pipeline, particularly concerning infectious diseases:

The following are recent recall updates posted by the US FDA.

In a very small international study using the antiviral, 68% of hospitalized patients on ventilators or oxygen showed improvement.

The therapy showed majority benefit in viral suppression at nearly 1 year among younger patients.