
From FDA recalls to a new COVID-19 variant, infectious disease news did not slow down in June. Catch up with Contagion's monthly recap of our most-clicked articles.

From FDA recalls to a new COVID-19 variant, infectious disease news did not slow down in June. Catch up with Contagion's monthly recap of our most-clicked articles.

We have developed our new Clinical Corner column and have other ways providers can get involved with Contagion.

Aerobic activity and muscle strengthening exercises reduced the risk of pneumonia or influenza mortality, even when the exercises were completed less frequently than recommended.

As the summer celebrations get underway, food safety remains vital to everyone.

The investigational therapy from Gilead showed effectiveness and safety at 96 weeks.

As of today, the new GSK and Pfizer RSV vaccines are authorized for adults 60 years and older. With CDC Director Dr. Rochelle Walensky’s endorsement, the vaccines are expected to be available as soon as this fall.

Receiving meals after hospital discharge significantly reduced the risk of 30-day rehospitalization and death in patients hospitalized with heart failure and other acute medical conditions.

Penicillin allergy is not uncommon, but its occurrence is exaggerated by erroneous allergy labeling that unnecessarily precludes treatment options.
New data from AIDSVu show significant disparities in the usage of HIV pre-exposure prophylaxis (PrEP). Black and Hispanic/Latinx Americans are underrepresented among PrEP users, despite accounting for a significant portion of new HIV diagnoses.
The Outpatient Automated Stewardship Information System (OASIS) is a platform that allows clinicians the ability to improve their prescribing practices in hopes of delivering a more judicious approach and moving towards stewardship.

There was a lower risk of Omicron infection for each 10-fold increase in preinfection IgG, and for each 2-fold increase in neutralizing antibody titers.

The strategy for administering beta-lactam antibiotics for treating critically ill patients with sepsis showed no clinical difference in a study of continuous versus intermittent administration of meropenem.
In Indiana, COVID-19 patients with chronic obstructive pulmonary disease, cardiovascular disease, and type 2 diabetes had significantly higher mortality rates than COVID-19 patients without these comorbidities.

The federal agency has identified a total of 5 cases in Florida and Texas within the last two months.

HIV testing is for everyone, explains Dr. Uri Belkind, regardless of sexual orientation, race, or gender.
As this annual day of recognition gets underway, free testing can be found throughout the United States.
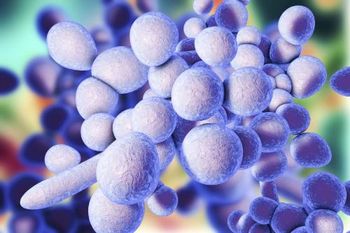
Candida auris, a drug-resistant fungal pathogen, has become a significant concern in Israel. A recent study reveals a 30-fold increase in C auris cases in 2021, with a strong correlation to COVID-19 surges.
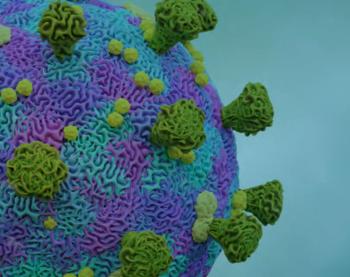
The strain, identified as EU11, accounts for 1.7% of cases nationwide in the United States.

Liver education has been void in the school systems and children and young adults stand to gain enormously when provided with awareness around this essential organ.

Clinicians should consider adenovirus testing in pediatric patients with hepatitis of unknown etiology. Here is an update on the incidence rates and lab diagnostics associated with identifying the virus.

Non-enveloped viruses were resistant to all types of wash products and duration tested, including synthetic soaps, such as the ones typically used in hospitals.

This week's most-read stories included a dive into racial disparities in hepatitis B and increased meningococcal disease in persons with HIV.
The film is part of a GSK campaign, Ask2BSure, that addresses this issue.

Even low levels of air pollution make COVID-19 worse, this study found. Air pollution exposure was associated with more severe disease, longer hospitalization periods, and an increased likelihood of intensive care admission.

The vaccine manufacturer has incorporated spike proteins for the XBB.1.5 sublineage of SARS-CoV-2 for its mRNA-1273.815 COVID-19 vaccine.
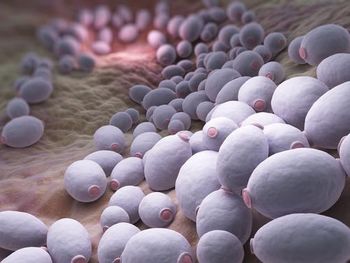
The CDC reports that during 2020-2021, the mortality rates for COVID-19—associated fungal Infections were significantly higher than non-COVID-19–associated fungal infections.

Older adults should receive the GSK or Pfizer RSV vaccine this fall, the CDC’s Advisory Committee on Immunization Practices (ACIP) voted yesterday. The recommendation is expected to be finalized by CDC director Dr. Rochelle Walensky.

Biopharmaceutical company, Vaccitech, has developed its VTP-300 vaccine, and is presenting a poster demonstrating positive early data at the ongoing European Association for the Study of the Liver (EASL) Congress 2023.
Though the drug is initially more expensive, treating C diff infection with fidaxomicin reduced costs overall by reducing CDI recurrences.
A company has developed an intelligence platform for better understanding influenza and other viral trends.