
Recently published data of bezlotoxumab dosing and safety in children with C diff was basis for expanding approval from just adults to as young as 1 years old.

Recently published data of bezlotoxumab dosing and safety in children with C diff was basis for expanding approval from just adults to as young as 1 years old.

With potential delays in treatment, bloodstream infections can be concerning for clinicians. Here is a review of the literature that offer guidance for treatment.

The Prescription Drug User Fee Act (PDUFA) action date is scheduled for the first quarter of 2024.

The agency says additional time is needed to decide upon a phase 4 program.

With recent reports of this vector-borne illness in the continental United States, there is concern this might become endemic in the country again.

Antibiotic actions on gut microbes of patient might affect the microbiome of housemates.

This week's news included managing infants with RSV, how COVID-19 surveillance has evolved, and looking at changing criteria for initial and sustained response to antibiotic treatment for C diff.
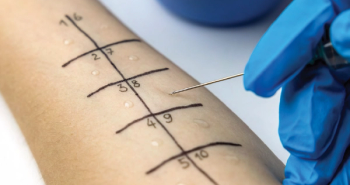
Patients with a penicillin allergy can be safely delabeled using minimal resources, opening up treatment options.

A clinician offers a glimpse of what it is like to treat infants with respiratory syncytial virus (RSV). And with the recent FDA approval of nirsevimab, how that will likely benefit families and the youngest population.
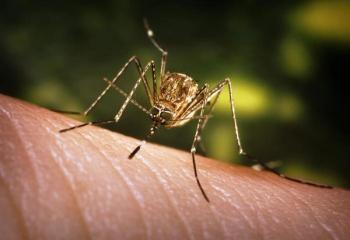
The vaccine is steps behind another Chikungunya vaccine candidate from Valneva, which is currently under priority review by the FDA.

Hepatitis B vaccine non-responders with chronic HCV infection were likely to respond and gain protection against HBV after HCV treatment.

Here is a review of the latest case loads from the World Health Organization (WHO).

Efforts to ramp up hepatitis B vaccination at birth and in infancy are crucial to controlling the disease in Africa, which accounted for 66% of new infections in 2019.

Surveillance data such as hospitalizations and wastewater metrics provide real COVID-19 prevalence numbers, but do we need to do more to be prepared ahead of another potential health crisis?
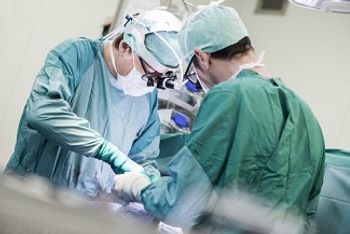
For patients undergoing surgery, looking at the potential benefits of this antimicrobial for prophylaxis.

HCV infections during pregnancy increased 16-fold from 1998 to 2018 in US, with adverse maternal and infant outcomes linked to opioid epidemic.

EXPAND Cdiff international group of infectious disease specialists propose changing criteria for initial and sustained response to antibiotic treatment.

From news about RSV to vaccines to an investigational hepatitis therapy, this week's Infectious Disease Update has something for everyone.
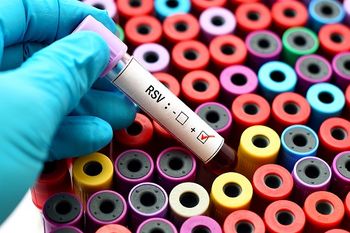
The lawsuit comes as the RSV market is likely to get more crowded, with a pending approval for Pfizer's pediatric indication later this month and a BLA submission from Moderna recently filed.

A new study confirms this form of surveillance to collect metrics for SARS-CoV-2 to understand infection prevalence on a bigger scale.

The study was designed to help scientists better understand how to prevent in-hospital transmission of C difficile.

The federal agency’s Advisory Committee on Immunization Practices (ACIP) also added the recently approved therapeutic to the Vaccines for Children program.

A new study demonstrated Moderna having a greater efficacy and slightly less adverse event profile in this patient population vs the Pfizer-BioNTech vaccine.
Nonsteroidal anti-inflammatory drugs can work with Clostridioides difficile toxins to target mitochondria of epithelial cells, leading to increased severity of infection that can be long-lasting.

Marrazzo is currently the director of the Division of Infectious Diseases at the University of Alabama at Birmingham, and will assume her role at the NIAID in the fall.
For people living with chronic hepatitis D, the latest study results confirm the benefit of injections of this first-in-class entry inhibitor.
The vaccine targets an additional 8 unique serotypes that disproportionally affect the adult population.

The CDC made this recommendation after reviewing relevant literature, and noting that complete testing jumped from about only two-thirds of patients to nearly all patients.
The vaccine has been approved since late 2019 for adults age 18 and older.
The first FDA-approved fecal microbiota product maintained a positive safety profile in the largest safety evaluation to date with up to 2 years of safety data.