
LMN-201, an oral biologic drug developed by Lumen Bioscience, has received Fast Track Designation from the FDA. The drug combines therapeutic proteins to neutralize the bacterium and toxin that cause C difficile infection.

LMN-201, an oral biologic drug developed by Lumen Bioscience, has received Fast Track Designation from the FDA. The drug combines therapeutic proteins to neutralize the bacterium and toxin that cause C difficile infection.

AI tools have taken front and center stage in 2023. One area of interest is how the new technology can be applied in infectious disease surveillance. A new article in the New England Journal of Medicine explores this concept.

The recommendations look to increase usage of the antiviral and decrease the routine use of the recombinant IL-1 inhibitor for COVID-19 treatment.

New findings from clinical trials reveal that Rebyota, a rectally administered treatment, demonstrates improved outcomes in patients with recurrent Clostridioides difficile infection (rCDI).

The presence of certain bacteria in the gastrointestinal tract may limit the effectiveness of antibiotic treatment for Clostridioides difficile.
Empiric therapy for severe Salmonella disease remains effective, but fluoroquinolone resistance calls for a shift towards trimethoprim-sulfamethoxazole as the preferred oral treatment for non-severe cases.

The mandatory reporting of Antimicrobial Use and Resistance (AUR) by hospitals participating in the CMS Medicare Promoting Interoperability Program beginning next year is a important strategy in curbing the overuse of antibiotics.

A study of patients with recurrent Clostridioides difficile infection (rCDI) found that Rebyota, a rectally administered live biotherapeutic, was considered easy, quick, and appealing due to the lack of bowel preparation.

There is a lot going on in this area in medicine and here is the most up-to-date news on health programs and therapies.

This study found a high proportion of reduced vancomycin susceptibility in C difficile patients, leading to lower rates of sustained clinical response.
A clinician presents data on this antibiotic for these troublesome infections at the ongoing MAD-ID conference.

From the relationship between nutrition and the gut to a newly approved C difficile therapeutic, these are the must-read stories in gastro health.

Sulbactam-durlobactam is efficacious against carbapenem-resistant infections, and Innoviva is preparing for the antibiotic's PDUFA at the end of this month.

There are currently no vaccines nor antiviral agents approved for RSV in infants. Sanofi presents data from the HARMONIE trial showing nirsevimab reduces hospitalizations due to RSV by 83.21%.

The latest issue of the Morbidity and Mortality Report discusses the cases.
A new study showed an introduction of beneficial microbiota.
Antifungal therapy was frequently initiated before essential diagnostic elements confirmed invasive fungal infections in critically ill COVID-19 patients.

Investigators found most cases were infected with adeno-associated virus 2 and a helper virus, though it’s unclear what role the former plays.
COVID-19 patients treated with the monoclonal antibodies tixagevimab and cilgavimab had few hospitalizations but no deaths.
Although vaccine effectiveness against the Omicron variant drops rapidly over time, a booster dose bolsters protection levels.

Adults who scored higher on the Healthy Eating Index were found to have higher gut microbiota diversity, as well as a higher exercise frequency and lower BMI.

This study determined the efficacy of face masks at reducing exhaled particles in children, as well as whether the type of activity affected the concentration and size of particles.

Ferring’s Rebyota therapeutic was studied in this population and demonstrated a 79% efficacy.

Investigators in Canada found no evidence to support extending treatment for Clostridioides difficile infection for patients on concurrent antibiotics

Ferring’s Rebyota, which was FDA approved late last year, demonstrated a high efficacy and favorable safety profile in the prevention of recurrent Clostridioides difficile.
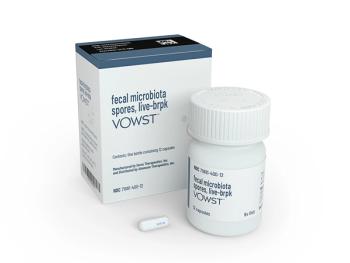
Recent phase 3 trial data for Vowst showed the microbiota-based therapeutic prevented recurrent C difficile infection in 91.3% of recipients after 8 weeks, a response that 94.6% of these patients maintained through week 24.
Steven Varga, PhD, explains why infants and young children are so susceptible to severe respiratory syncytial virus (RSV) infections.

The Committee for Medicinal Products for Human Use expressed a positive opinion for bulevirtide for treatment of hepatitis D (HDV).

Carlos Del Rio, MD, updates clinicians and the public on where we are now on the respiratory virus and the modalities to treat and prevent severe disease.

A clinician provides insights on this significant topic as well as resources in finding information on them.