This therapy showed a 48% reduction in severe respiratory disease.
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

This therapy showed a 48% reduction in severe respiratory disease.

While social determinants, stigma about the virus, and continuum of care issues remain challenges to many people with HIV, The IDSA is looking to address these issues with its new guidance.

The FDA’s breakthrough designation of cabotegravir along with adaptive strategies being deployed are the biggest stories in HIV prevention this year.
A principal investigator studying dolutegravir/lamivudine discusses its efficacy and potential treatment benefits.

The authorization recommends dosage intervals of between 4 and 12 weeks.

Trial to enroll up to 30000 volunteers across approximately 115 sites in the US and Mexico.
With the public beginning to get vaccinated, CDC published information for people in some medical categories and considerations on severe allergic reactions.
The new COVID-19 variant, B.1.1.7, continues to make its way around Europe and the world with new cases.
Vaccine manufacturer Valneva recently announced it was initiating a phase 1/2 trial in the United Kingdom.

Review of data in trial registries shows they specifically excluded this group from studies involving therapies.
Covaxx CEO Mei Mei Hu has seen vaccines from development all the way through to government approval. She offers her unique perspective into vaccine creation and an update on her company’s COVID-19 vaccine.

In a mature market of agents, the investigational therapy, ibrexafungerp, from Scynexis is looking at a few antifungal indications with a potential FDA approval in 2021.

This non-prescription test allows users to perform and get results themselves without a medical provider or laboratory needed.

Just one day before the FDA meeting to discuss potential US authorization of the Pfizer and BioNtech vaccine, that country approves the vaccine.

Tonix Pharmaceuticals is developing a horsepox virus vector vaccine based on a historic platform which was first used for smallpox, and applying it to the world’s most recent virus.
Cincinnati, Ohio-based Blue Water Vaccines is working on developing a vaccine that will permanently protect against current and future influenza strains.
While the antibodies declined slightly at 3 months, all participants showed elevated levels.

The agency adds 2 options lowering the number of days to isolate.

The Advisory Committee on Immunization Practices (ACIP) met yesterday and decided that healthcare workers and residents in long-term care facilities will be prioritized as the first people eligible for the impending COVID-19 vaccines.

The companies are ready to ship doses immediately.
Mady Hornig, MA, MD, discusses the mental and emotional aspects of dealing with a diagnosis and the evolution of how people are processing the virus many months later after it started.

A team at Columbia University's Mailman School of Public Health is partnering with a chronic fatigue syndrome (CFS) organization to study COVID-19 and CFS.

A team at Columbia University's Mailman School of Public Health is looking at the long term of effects in some patients who are suffering from this phenomenon.

Mady Hornig, MA, MD, shares her experiences of having lingering effects as part of a phenomenon where some people suffer from the virus over many months.
OncoSec recently received an FDA IND clearance to begin a phase 1 trial.

This CDC panel discussed who should be vaccinated first and the flu-like effects from the vaccines.

Data shows their AZD1222 vaccine met its endpoint and they are preparing regulatory submission for conditional or early approval.
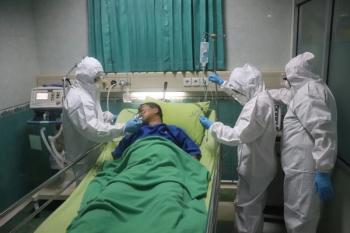
This EUA is for the treatment of hospitalized patients who require oxygen.

Phase 2 results include a large cohort of seniors in the study.

The investigational vaccine is being reported as 95% effective.