Antibiotics
Latest News

Latest Videos

CME Content
More News

Zevtera approval shows efficacy and marks a new treatment option for bacterial infections including MRSA.

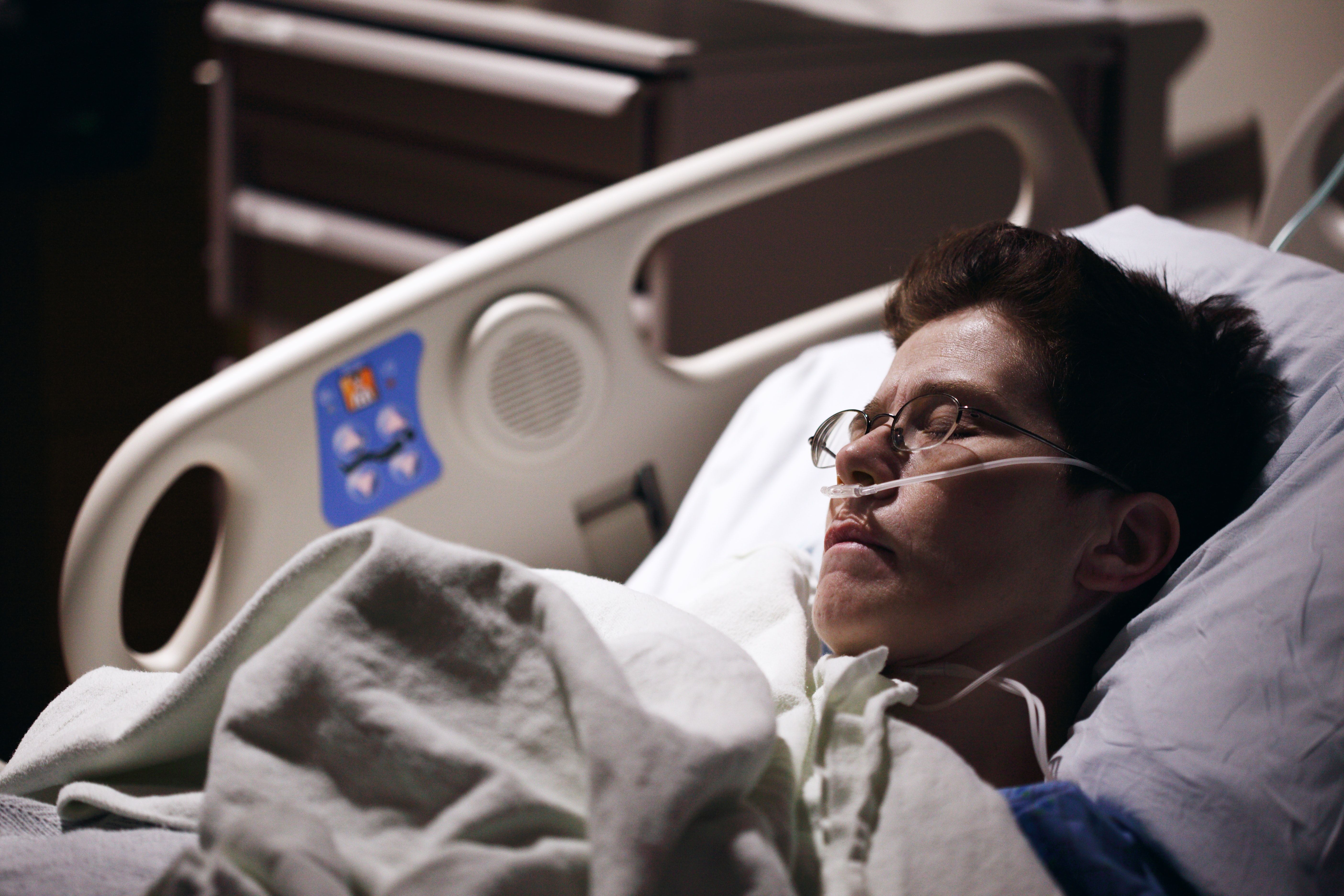
Here is a case study involving a patient with the virus and the clinical approach in thinking about appropriate treatment while keeping stewardship in mind.
Clinicians review a patient case including diagnosis, treatment, and follow-up.
In today’s world of sophisticated, complex tests, an evolving relationship between laboratory professionals and clinicians can aid in providing a quicker diagnosis and help to achieve better patient outcomes.

There is value in leveraging the contributions of nurses, laboratorians, and informaticists in antimicrobial stewardship.
A cautious approach for now is warranted as well as a need for a randomized clinical trial.

Investigators find that bacteremia rarely develops from asymptomatic bacteriuria, and that empiric antibiotic treatment should be reserved for those at risk.
Here is a review of therapies for treating this bacterium.
Low-frequency resistant cells in bacterial isolates are challenging to detect and may contribute to unexplained treatment failure.

In the latest issue, Editor-in-Chief Jason Gallagher, PharmD, FCCP, FIDP, FIDSA, BCPS, discusses the continuous antibiotic trade-off of targeted vs broad spectrum therapies and cheap vs expensive options as they relate to UTI treatment.

The OASIS platform will help outpatient facilities use prescribing data to improve patient care.

A team developed and validated a new analytic method to quantify omadacycline and its epimerization in stool to facilitate microbiome research.

Management of this condition has been associated with antibiotic misuse. In the latest Bench to Bedside column, clinicians offer insights on therapy indications and what the latest literature reports on the condition.

A new paper outlines how these variables can be connected to create situations where patients get less-than-optimal outcomes.

Cefepime-enmetazobactam (Exblifep, Orchid Pharma) was given the federal nod for the indication of treating complicated urinary tract infections (cUTI) in adults.

The federal agency did not request further trials for the investigational therapy, cefepime-taniborbactam, but wanted more information about its chemistry, manufacturing, and controls.

Eric D. Donnenfeld, MD, discusses 3 uncommon yet highly infectious eye diseases, emphasizing the importance of timely treatment and preventative measures.

New review article outlines the evidence and future work that needs to be done with regards to doxycycline post-exposure prophylaxis (doxy-PEP).
A clinician reviews the literature and offers some insights on the role of this antibiotic in bone, joint, and hardware infections.

A recently published study demonstrated the superior efficacy of cefepime–taniborbactam over meropenem for managing complicated urinary tract infections, suggesting a promising treatment option amid rising antibiotic resistance challenges.

Inhaled nanoparticles deliver antibiotic and antimicrobial peptides through mucus and biofilm barriers in animal model of infection-exacerbated COPD.

ePOCT+, a digital clinician decision support tool, reduced outpatient antibiotic prescribing without adverse health outcomes, potentially mitigating development of antimicrobial resistance.
New vancomycin dosing regimens are proposed to improve outcome and minimize toxicity in overweight and obese patients with renal insufficiency.
New challenges in the fight against gonorrhea in the US.

Emerging treatment options for patients with persistently positive blood cultures with Methicillin-Susceptible Staphylococcus aureus (MSSA).