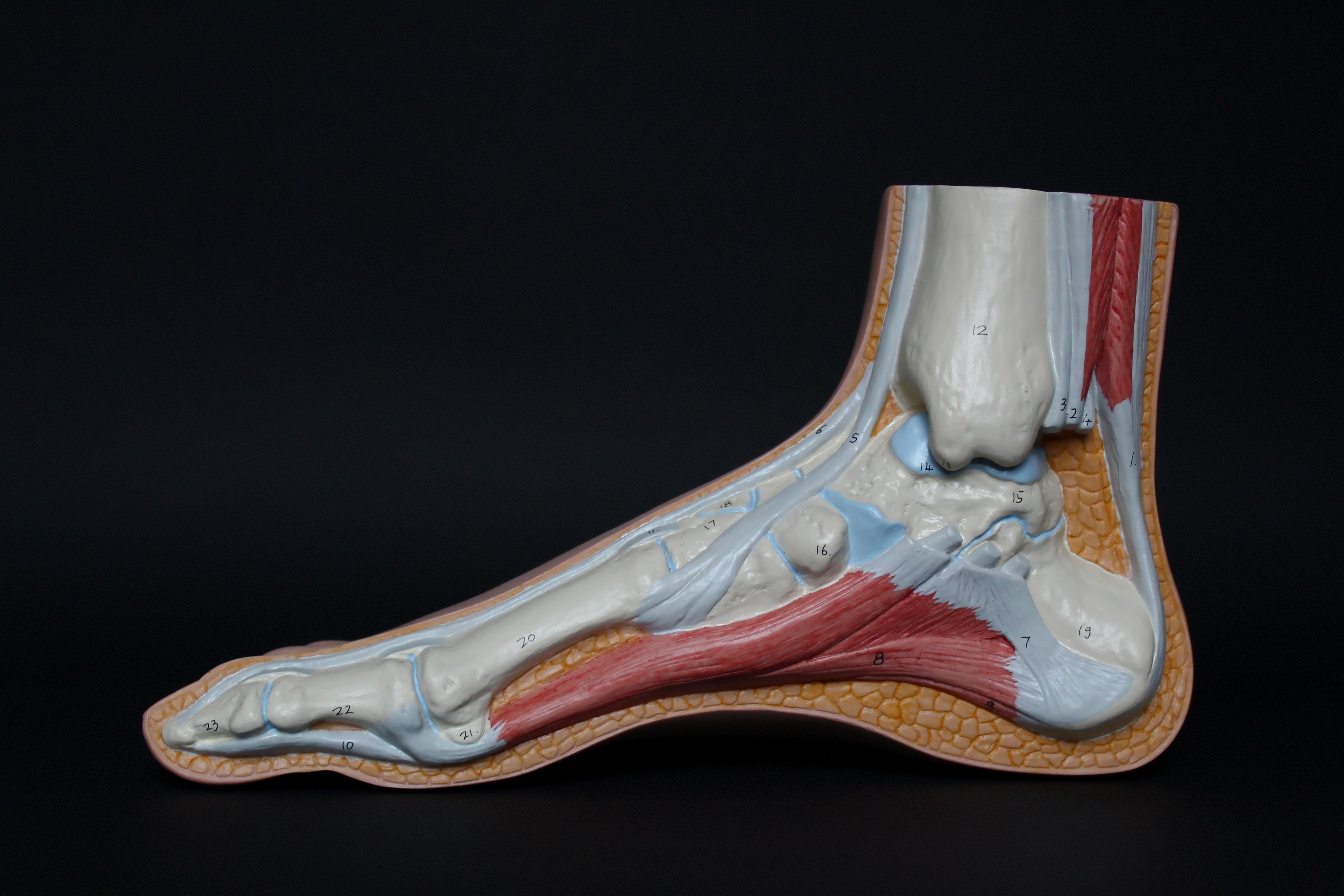
Antibiotics
Latest News
Latest Videos

CME Content
More News

A study demonstrated patients were frequently prescribed broad-spectrum antibiotics when more narrowly tailored antibiotics were considered the optimal choice.

A third to a half of PPIs are overprescribed, potentially leading to an increased risk of the colonization with drug-resistant microorganisms.

This study analyzed concerns that a lack of time may lead primary care physicians to prescribe unnecessarily as a “quick fix.”

The congressional bill has been on Capitol Hill for over 2 years now, and the interested stakeholders are continuing their lobbying efforts to get it passed.
A single oral dose of azithromycin reduced the risk of maternal sepsis or death by 33% in women who delivered vaginally.

Antimicrobial stewardship programs can protect drugs listed on the WHO Watch List, but the benefits are also dependent on countries' income level.

An increase in extensively drug-resistant Shigella infections prompted the CDC to release a health advisory for this major cause of bacterial diarrhea.
"This case gives uncomfortable insight into a gap in early detection of infections in immunocompromised, high-risk patient populations. "
Since the vancomycin guideline update, institutional leaders have been faced with the challenge of implementing and monitoring AUC-guided dosing.
Carbapenem-resistant Enterobacterales present considerable treatment challenges. Recent cumulative evidence supports preference for therapies.

Although fecal microbiota, live-jslm (Rebyota; RBX2660) is the first fecal microbiota product approved by the FDA, there are other microbiota-based agents in the pipeline.

Both undertreatment and overtreatment were common in this cohort of penicillin-allergic pregnant women with Group B Streptococcus (GBS).

Linezolid Coadministration With Medications for Opioid Use Disorder: A Serotonin Toxicity Tightrope?
This study characterized the incidence of serotonin syndrome and identified contributing risk factors with combined medications for opioid use disorder (MOUD) and linezolid use.

Six respiratory infections are among the most common diagnoses for which antibiotics often are inappropriately prescribed, and new research sheds light on the effects, both in increased risk of adverse events and excess health care costs.

Bacterial co-infections with COVID-19 are uncommon, but frequent use of antibiotics has fostered treatment resistance in co-occurring and secondary infections.

Antibiotics are apparently not the only widely used pharmaceuticals that can induce emergence of treatment resistant bacteria.
Looking to appeal to the financial incentives of antibiotic development, a recent policy paper explores how lives can be saved and a return of investment (ROI) can be realized.

Can a similar approach that brought breakthrough therapies for cystic fibrosis bring about much needed change to antibiotic development?

Patients treated with SER-109 reported a significant improvement in disease-specific health-related quality-of-life, regardless of their clinical outcome.

The investigational therapy developed by Entasis is indicated for treatment of Acinetobacter baumannii.

If approved on its March PDUFA date, rezafungin could be the first new Candida treatment option in over a decade.

An analysis of the actions taken to combat antimicrobial resistance in 114 countries finds national action plans easier to develop than to implement.

It's Friday the 13th, but you can counteract bad luck by catching up on this week's top infectious disease news.
A 2-year retrospective study looking at patients admitted to the ICU showed that the number of intubated males infected with Acinetobacter baumannii (AB) was double the number of intubated females.

It's your day, pharmacists! Here's a roundup of our top 5 stories by infectious disease pharmacists, for infectious disease pharmacists.