
Symptoms of COVID-19 can persist for months in about a third of cases, including among those with mild illness, a new study confirms.

Symptoms of COVID-19 can persist for months in about a third of cases, including among those with mild illness, a new study confirms.

The VRBPAC voted 22-0 with no abstentions to support the benefit-risk profile associated with Ad26.COV2.S on Friday night.

Vitamin B6 is a water-soluble vitamin found in various foods such as fish, whole grains, and bananas.

The US Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) recommendation paves the way for the authorization.
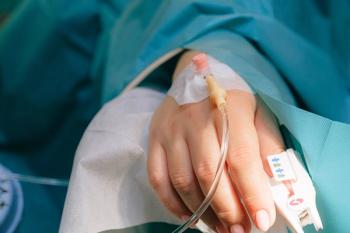
Patients with cardiometabolic conditions have a high risk of poor outcomes.

The country has no domestic production, and the government has been accused of not acquiring doses quickly enough.
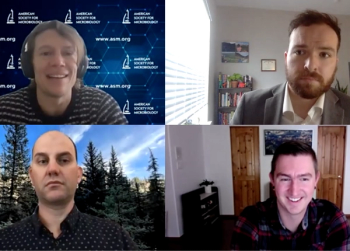
A team of cross-country investigators discuss their coordinated tracking of a new variant in the US earlier this year.

Multiple observational and ex-vivo laboratory studies have demonstrated that IL-6 is an important cytokine associated with disease severity and mortality.

If approved, the decision could have a significant impact on the mass vaccination programs.

In a phase 3 study, rheumatoid arthritis (RA) therapy, tocilizumab, did not improve clinical status or lower mortality in these patients.
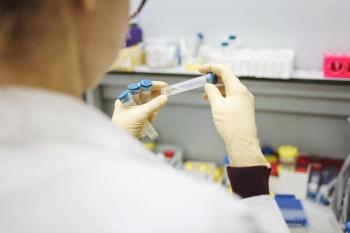
Pooled testing allows multiple samples to be processed at once and help increase testing efficiency.

Vaccine clinical trials have fallen short of diversity guidelines during the past decade, with minority groups underrepresented and key data missing in many trials, a new study found.

The new trial comes at a time when the FDA has provided guidance on navigating immunogenicity trials for vaccines designed against more transmissible SARS-CoV-2 strains.

Study results highlight that new data is constantly emerging and that society must be flexible in its response to the pandemic.

A massive assessment of a quickly vaccinated population shows the mRNA vaccine provides greater protection over time.
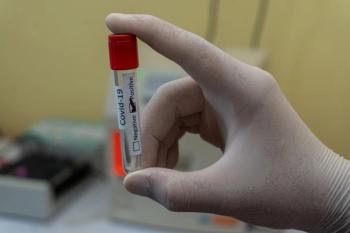
Not every positive patient missed by PCR and antigen tests will be infectious to others.
The data supports strong guidance for enhanced precautions to prevent SARS‐CoV‐2 infection in pregnancy.
A better option is needed that can incorporate specific mortality-related variables, the study authors said.

Supporting EUA data for the one-shot vaccine candidate showed Americans fared best in a 40,000-participant, international trial.
Investigators sought to compare trends in US pediatric hospitalizations in 2020 with those the decade prior to COVID-19.

SAP1 and SAP6 were found to inhibit a SARS-CoV-2 infection.

Understanding underlying immunological mechanisms can provide critical insights.

This partnership of recognized experts provided feedback about a variety of topics related to the virus.

The new advisory is informed by currently-understood effect of authorized vaccines against circulating SARS-CoV-2—which show benefit nonetheless.

According to the federal agency, anaphylaxis from mRNA COVID-19 vaccinations is a rare event.