
Biopharmaceutical company, NeuroRx, reported positive data from its phase 2/3 trial for its therapy, aviptadil acetate (Zyesami), in patients with severe COVID-19.

Biopharmaceutical company, NeuroRx, reported positive data from its phase 2/3 trial for its therapy, aviptadil acetate (Zyesami), in patients with severe COVID-19.
India recorded over 100,000 cases on Sunday, the countries highest daily total ever.

An expert stresses the need to consider individuals' worry over information—and even their history with systemic biases.

Vaccine access and new cases are both increasing. An expert discusses earning trust in at-risk communities.

The screening methods used in the study proved to be reliable and inexpensive alternatives to the current standard used in testing.
Health regulators in the UK have now found 30 cases involving rare blood clot events after the administration of the AstraZeneca COVID-19 vaccine, but still recommend its use.
Hospitalized patients with COVID-19 and chronic inflammatory diseases were seen to have no statistically significant increased length of hospital stay or need for mechanical ventilation.

The federal agency said people who are fully vaccinated “are less likely to get and spread COVID-19,” and can travel safely in the United States.

The 2 revisions made to the EUA pertain to the number of doses allotted to the current vials containing the vaccine, and allow a new vial that has a maximum of 15 doses.

Investigators discovered that the variant was in many other countries before it was discovered and created a tool to help detect emerging variants in the future.

With the small size and portability of NIRVANA, the test could be used for fast virus detection at various locations and could be used to monitor wastewater or streams for the presence of new viruses.

A manufacturing plant that was set to produce the Janssen (Johnson & Johnson) vaccine mixed up the ingredients of 2 vaccines.

To date, there have been only a few small reports of seizures in patients with severe COVID-19 and the effects them on patients' health was previously unknown.

The joint World Health Organization (WHO)-China Report looked at various scenarios and its theory of an animal to animal to human transmission.

TOP1 inhibitors can limit the expression of hyper-inflammatory genes in the lungs of infected animals and improve outcomes days after the initial infection.

The pivotal phase 3 data, based off 18 reported cases among placebo patients, includes the companies' intention to request authorization for adolescents aged 12-15 years old.

The technique will be applicable to a broad set of other diseases and can give doctors a practical method for delineating important differences within disease categories.
President Biden has increased vaccine doses to pharmacies to help speed up inoculations in light of recent infections.

The diagnostic testing kit is easy to use at home and will eventually be able to pick up the thousands of variants of COVID-19.

The country’s federal health agency decided upon this action based on the small number of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) incidents in Europe, which are being investigated.

The coronavirus disease 2019 pandemic hurt the bottom lines of airlines, as the public worried air travel would expose them to catching the virus. A new analysis suggests how airlines can best mitigate that risk.

Cases have increased by 16%, while 50 million have now been fully vaccinated. Federal leadership is asking Americans to hold on longer.
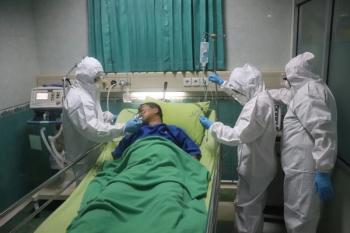
Intermediate-dose prophylactic anticoagulation did not improve mortality or venous or arterial thrombosis in patients with COVID-19 in the ICU compared with standard dosing, a new study found.

The Moderna and Pfizer-BioNTech vaccines are highly effective and transmission is unlikely.

England starts easing lockdown restrictions in hopes of removing most by early summer.